The WHO has established that coronary artery and cerebrovascular disease represent the main causes of premature death and disability in developed countries.1 However, the incidence and prevalence of cardiovascular and cerebrovascular disease is also increasing in developing countries as a result of population ageing and changes in lifestyle. For these reasons, the prevention and treatment of cardiovascular disease continues to be one of the key objectives of global health policies. Health initiatives aimed at raising awareness about heart-healthy lifestyle habits and at controlling risk factors such as smoking, dyslipidaemia or arterial hypertension (AHT) are essential strategies in these campaigns. However, a better understanding of the causes of cardiovascular disease, as well as the identification and treatment of new risk factors, is also urgently needed. Recent evidence indicates that obstructive sleep apnoea (OSA) may be a potentially modifiable risk factor for vascular disease.
Sleep Apnoea Syndrome and Cardiovascular Disease
Epidemiology, Diagnosis and Treatment
OSA is a disorder characterised by snoring and presence of apnoea or obstructive hypopnoea due to the collapse of the upper airway (either partial or complete), accompanied by hypoxia, during sleep. Most of these respiratory disturbances during sleep cause oxygen desaturations and resaturations and changes in intrathoracic pressure; they end with a micro-awakening, with the resulting fragmentation of sleep and the appearance of excessive daytime sleepiness, which is the most frequent symptom of this disorder. These episodes are assessed by using polysomnography to measure the apnoea–hypopnoea index (AHI), with an AHI ≥5 events/h being considered pathological. The AHI is used to define disease severity: scores of 5–14.9 are considered mild; scores of 15–29.9, moderate; and scores ≥30, severe. The prevalence of OSA has risen over time and varies according to study: depending on the cut-off point of the AHI used, it is currently estimated to affect 10% of men and 15% of women.2 The main risk factors for OSA are obesity, craniofacial or oropharyngeal anatomical abnormalities, male sex and smoking. It is important to stress that in subjects with several cardiovascular risk factors the prevalence of asymptomatic OSA is higher than in healthy adults of the same age and sex, and is unrelated to BMI. Consequently, if we take drowsiness or obesity as defining features of the OSA phenotype, its prevalence in patients with cardiovascular disease may be underestimated.3–5 Given that obesity is an important causative factor, the prevalence of OSA is likely to continue rising in line with the obesity rate. Diagnosis and treatment of OSA have increased in the last 5–10 years, but current evidence indicates that the disease remains undiagnosed in a high percentage of patients.6,7
The first-line treatment for all patients with OSA is continuous positive airway pressure (CPAP). This technique provides pneumatic splinting of the upper airway, thus reducing airflow obstruction and apnoeic events. CPAP improves self-reported sleepiness and quality of life, but patient non-adherence may limit its clinical benefit.8
OSA and Cardiovascular Risk
Studies in recent years have shown that OSA is an independent risk factor for hypertension and increases the risk of cardiovascular disease. The relationship between OSA and cardiovascular disease can be attributed, at least in part, to the coexistence of cardiovascular risk factors in the two pathologies such as sex, age, overweight, alcohol consumption, tobacco and sedentary lifestyle.
The current evidence linking OSA with increased cardiovascular risk is strong. In this study, a search was performed to identify papers on the association between OSA and new-onset hypertension and cardiovascular adverse events in the PubMed database (MeSH: medical subject headings) using the following search strategy: ‘sleep apnoea, obstructive’ [MeSH] and ‘cardiovascular diseases’ [MeSH], with ‘hypertension’, ‘cardiovascular adverse events’, ‘atrial fibrillation’, ‘ischemic heart disease’, and ‘cardiovascular mortality’ as key words. The search was limited to prospective studies and recent meta-analyses. To assess the efficacy of CPAP for the primary and secondary prevention of cardiovascular adverse events, the following search strategy was applied: ‘sleep apnoea, obstructive’ [MeSH] and ‘continuous positive airway pressure’ [MeSH] and ‘cardiovascular diseases’ [MeSH] only in randomised controlled trials. After the electronic search the references cited were also reviewed.
Observational (cross-sectional and longitudinal) epidemiological studies have consistently reported higher cardiovascular-related morbidity and mortality rates in patients with severe untreated OSA than in patients undergoing CPAP or those who do not present severe OSA.9–12
Hypertension and Cerebrovascular Disease
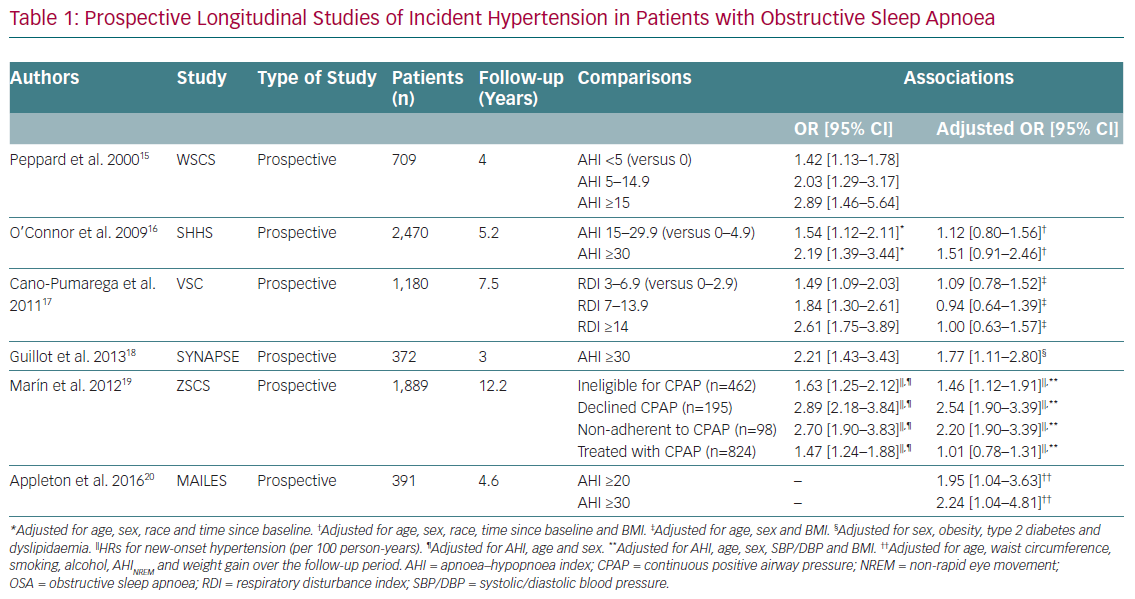
AHT is the cardiovascular disease most clearly linked to OSA. Cross-sectional population-based studies have consistently found higher rates of hypertension in patients with OSA than in controls, independent of potential confounders such as obesity and age.13 The Sleep Heart Health Study (SHHS) was designed to investigate the relationship between OSA and cardiovascular disease in 6,424 individuals (mean age 65 years) obtained from several cohorts of epidemiological studies of cardiovascular disease in the US.13 All subjects had cardiovascular risk factors and a complete sleep study at baseline. A linear relationship was observed between the severity of OSA, measured on AHI, and the prevalence of AHT, regardless of other factors.13 Approximately 50% of patients with OSA have high blood pressure, and up to 71% of patients with resistant hypertension have a diagnosis of OSA.14 Prospective clinical studies also demonstrate a high incidence of hypertension in patients with OSA and normal blood pressure at baseline, especially in those with severe OSA (Table 1).15–20 Recent meta-analyses suggest a dose–response relationship between OSA severity and the risk of essential hypertension, especially in male Caucasian patients.21,22
Other controlled studies have evaluated the effect of CPAP on blood pressure in patients with OSA and have demonstrated a significant reduction, suggesting a better response in subjects with severe OSA and excessive daytime sleepiness, resistant AHT, and greater adherence. Effective treatment of OSA, especially with CPAP, reduces blood pressure regardless of the baseline figures.23–25 The reduction in blood pressure with CPAP treatment is slight, but significant. In a meta-analysis of 1,820 OSA patients, CPAP treatment was associated with a significant reductions of 2.6 ± 0.6 mmHg in systolic blood pressure and 2.0 ± 0.4 mmHg in diastolic blood pressure.26 The reduction in blood pressure with CPAP is lower than with antihypertensive drugs, but their combined use in patients with resistant AHT can help to reduce it at a later stage.27 The antihypertensive effect of CPAP is greater in patients with more severe OSA and in patients with excessive daytime sleepiness.28,29
Cardiovascular Disease
There is also evidence of a relationship between OSA and the development and progression of ischaemic heart disease (IHD), heart failure and arrhythmias.
The prevalence of OSA in patients with IHD is higher than in the general population, and up to 70% of patients with acute coronary syndrome (ACS) have undiagnosed OSA. The presence of calcifications in the coronary arteries, a subclinical marker of atherosclerosis, was recorded in 67% of patients with OSA versus in 31% of patients without OSA.30 In addition, the SHHS demonstrated that the presence of obstructive apnoea increases the risk of CHD by 30%.13
OSA can induce IHD by various mechanisms: among others, by favouring coronary atherosclerosis through oxidative stress, systemic inflammation and endothelial dysfunction due to intermittent hypoxia. In addition, the increase in platelet activity and aggregation, as well as the reduction in fibrinolytic activity, together with the increase in fibrinogen in patients with OSA may favour coronary artery thrombosis, a pathogenic mechanism of ACS.
The presence of OSA may worsen the prognosis of existing IHD. Several prospective observational studies conducted in patients with known IHD have observed an increased risk of cardiovascular events, including recurrent events, in patients with OSA versus subjects without OSA. In a multicentre study that included 1,311 patients undergoing percutaneous revascularisation with a follow-up of 1.9 years, patients with moderate or severe OSA (AHI ≥15) had (regardless of other confounding factors) a 1.5-fold higher risk (adjusted HR 1.57; 95% CI [1.10–2.24]; p=0.013) of an adverse cardiovascular or cerebrovascular event than revascularised patients without OSA.31 OSA has been identified as a possible risk factor for nocturnal cardiac ischaemic events. One study of patients with OSA and ACS reported the occurrence of ACS between 12 am and 6 am in 32% of cases, compared with only 7% in patients without OSA.32
The possible effects of OSA on the recovery of left ventricular systolic function after MI are controversial. It has been reported that, after acute MI, the presence of OSA compromises the recovery of left ventricular function by increasing and remodelling the area of extension of myocardial necrosis.33,34 The risk of recurrence of cardiovascular events after a heart attack is higher in patients with excessive daytime sleepiness, regardless of AHI and oxygen desaturation.35
OSA may induce or worsen an existing heart failure via various mechanisms regardless of the presence or absence of hypertension. The prevalence of OSA in patients with heart failure and reduced or preserved ejection fraction ranges from 11% to 55%, and the degree of dysfunction seems to be associated with the severity of OSA.36 Conversely, the prevalence of heart failure in patients with OSA may be more than twice that in patients without OSA.37 The SHHS noted an independent association between OSA and the risk of developing heart failure.13,38 A controversial issue is whether OSA increases mortality in patients with heart failure: some studies have found that OSA is an independent risk factor for mortality in patients with heart failure, whether ischaemic or not, while other studies have not demonstrated this association.39–41
Heart rhythm disorders have been described in patients with OSA, the most frequent arrhythmias being atrial and ventricular extrasystoles, sinus arrest and atrioventricular conduction block.42 Bradyarrhythmias are usually due to vagal activation that occurs at the end of the apnoeas mediated by hypoxic stimulation on the carotid body, and so the degree of bradycardia is linked to the severity of OSA and especially to the degree of hypoxaemia. Other types of arrhythmia such as AF or ventricular tachycardia usually occur in the context of an associated structural cardiac injury. However, the prevalence of cardiac arrhythmias in patients with OSA is not well known. In the SHHS, the presence of severe OSA (AHI ≥30) was associated with a fourfold increase in the risk of AF, a threefold increase in non-sustained ventricular tachycardia and a twofold increase in complex ventricular extrasystoles compared with subjects without OSA (AHI ≤5).43 In that study the risk of an arrhythmic episode was found to be 18-fold higher during a respiratory event than during normal breathing. A decrease in nocturnal oxygen saturation has been identified as a predictor of AF, independent of obesity.44 Patients with untreated OSA have a higher risk of recurrence of AF after cardioversion than treated patients, and an increased risk of pulmonary vein ablation failure.45 However, several studies demonstrate a higher prevalence of AF in patients with central and non-obstructive apnoeas, suggesting that it is heart failure, rather than OSA, that plays the leading role in this association.46
Cardiovascular Mortality
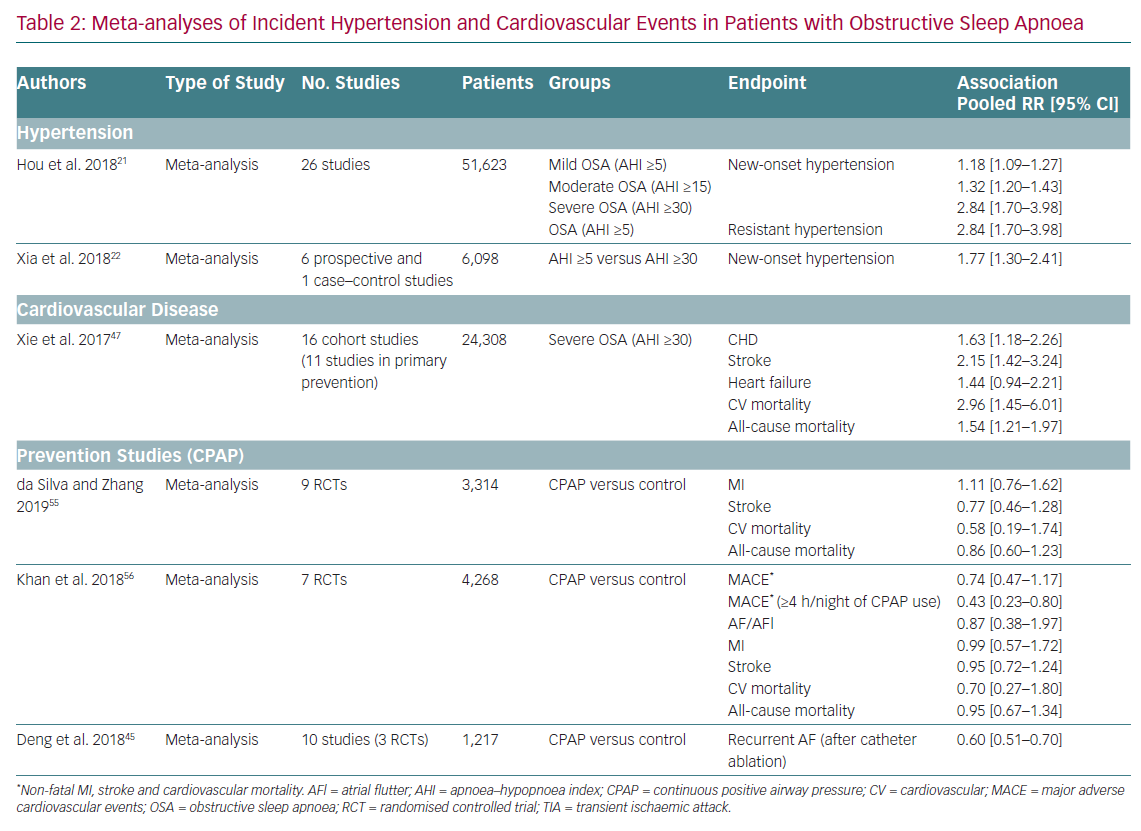
Given the relationship between OSA and its possible clinical consequences, an association between OSA and mortality might be expected. There is sufficient evidence to state that severe OSA (AHI ≥30) is an independent risk factor for mortality. Cross-sectional and prospective population-based studies have also found an increase in cardiovascular mortality in patients with severe untreated OSA or with insufficient use of CPAP. A meta-analysis of cohort studies involving 24,308 participants found that the risk of cardiovascular mortality was threefold higher in patients with severe OSA (AHI ≥30) than in the control group without OSA (HR 2.96; 95% CI [1.45–6.01]; Table 2).47
Clinical Trials in Primary and Secondary Cardiovascular Prevention
Clinical trials aiming primarily to assess the effect of CPAP treatment on the development of serious cardiovascular events associated with OSA are relatively recent, such that until now almost all the evidence came from observational studies. Several of these studies have found that treatment with CPAP reduces cardiovascular morbidity and mortality (defined as a set of different cardiovascular events including death due to cardiovascular causes, stroke, or acute MI).
Primary Cardiovascular Prevention
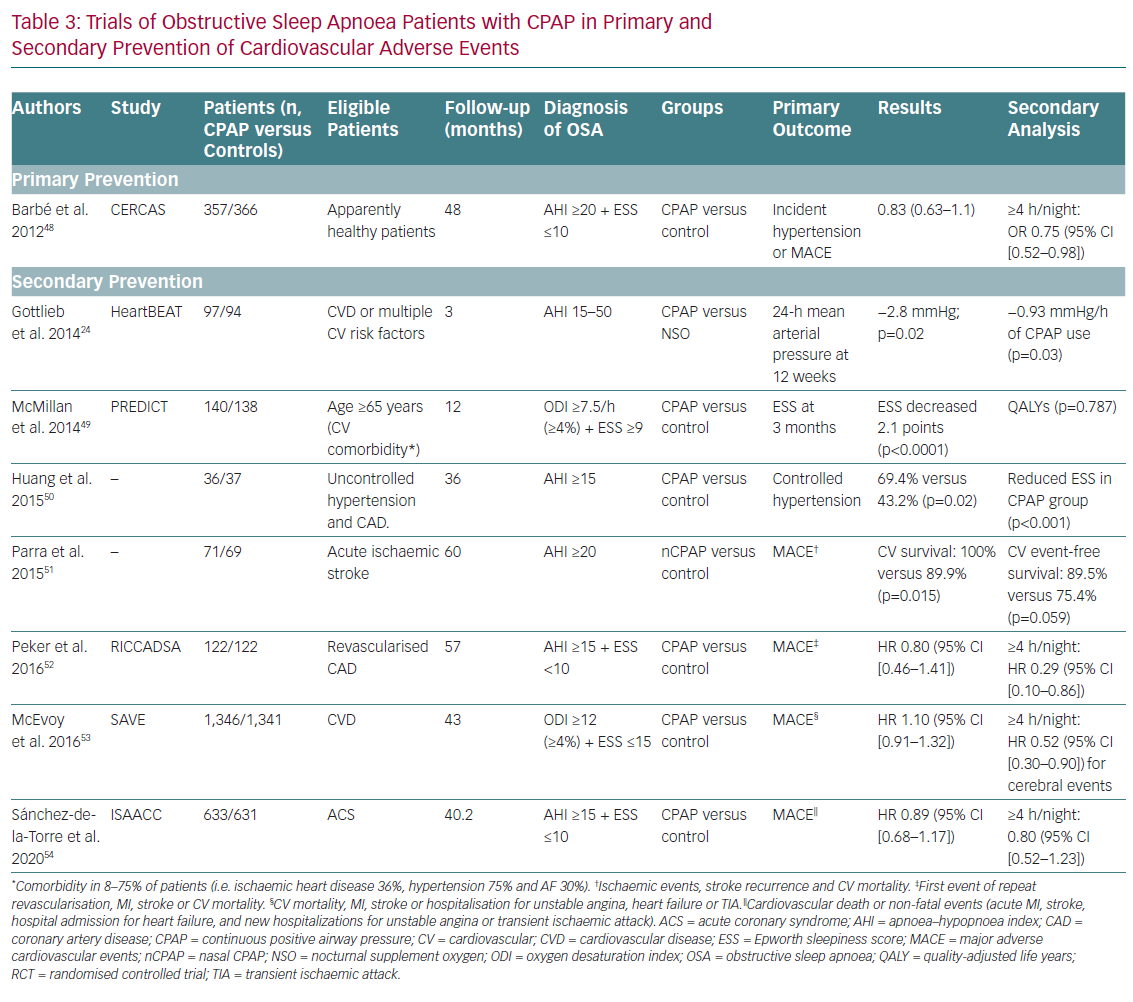
The study by Barbé et al. (the Spanish Sleep and Breathing Network; CERCAS) analysed 725 patients with moderate or severe OSA (AHI ≥20 on polysomnography or cardiorespiratory polygraphy) without daytime sleepiness (Epworth score ≤10) and without previous cardiovascular events.48 The authors studied the effect of CPAP on primary cardiovascular prevention by analysing the incidence of AHT (need for antihypertensive drugs or blood pressure >140/90 mmHg) or the development of non-fatal MI, non-fatal stroke, transient ischaemic attack (TIA), hospitalisation for unstable angina or arrhythmia, heart failure or cardiovascular death with a mean follow-up of 4 years (IQR 2.7–4.4 years). No differences were observed between patients randomised to CPAP and those to conservative treatment in terms of the incidence of AHT or cardiovascular events. However, a post-hoc sub-analysis suggested that CPAP reduces the incidence of AHT and cardiac events in patients with adherence to treatment ≥4 h/night (incidence density ratio 0.75; 95% CI [0.52–0.98]).
Secondary Cardiovascular Prevention
The effect of CPAP treatment on cardiovascular secondary prevention in patients with OSA has been analysed in other randomised clinical trials (Table 3).24,49–54 In the Randomized Intervention with Continuous Positive Airway Pressure in Coronary Artery Disease and Obstructive Sleep Apnea (RICCADSA) study, 244 patients with moderate or severe OSA (AHI ≥15) without daytime sleepiness (Epworth score <10) underwent coronary revascularisation and were randomised to CPAP or conservative treatment for 57 months.52 On intention-to-treat analysis, there were no differences between the two groups in the combined primary cardiovascular endpoint of repeat revascularisation, MI, stroke and cardiovascular mortality. However, patients with adherence ≥4 h/day had a lower cardiovascular risk than untreated patients or those receiving CPAP <4 h/day (HR 0.29; 95% CI [0.10–0.86]). As with the results on the efficacy of CPAP in primary cardiovascular prevention, poor adherence to CPAP treatment affects therapeutic response in patients with OSA.
The Sleep Apnea Cardiovascular Endpoints (SAVE) study randomised 2,717 patients aged between 45 and 75 with established cardiovascular or cerebrovascular disease and moderate or severe OSA to usual care or usual care plus CPAP.53 Moderate or severe OSA was defined as an oxygen desaturation index (number of times per hour that the oximetry recording detects a decrease in arterial oxygen saturation ≥4 percentage points from baseline) of at least 12, determined using a home sleep study device (ApneaLink, ResMed). The primary composite endpoint included death from cardiovascular cause, MI (including silent MI), stroke, hospitalisation for heart failure, acute coronary symptoms (including unstable angina) or TIA. The use of CPAP did not significantly reduce recurrent serious cardiovascular events in the intention-to-treat analysis, despite the significant reduction in daytime sleepiness and improved quality of life. However, propensity score-matched analyses showed that patients with good adherence to CPAP treatment (≥4 h/day) had a lower risk of stroke compared with patients with conventional treatment (HR 0.56; 95% CI [0.32–1.00]), as well as a lower risk of the non-pre-specified composite endpoint of cerebrovascular events (HR 0.52; 95% CI [0.30–0.90]).
The recently published Continuous Positive Airway Pressure in Patients with Acute Coronary Syndrome and Obstructive Sleep Apnea (ISAACC) study included 2,834 patients with ACS who underwent respiratory polygraphy.54 Of these, 1,264 had moderate or severe OSA (AHI ≥15/h as measured on cardiorespiratory polygraphy) and were randomly assigned to receive CPAP or usual care. The 1,287 patients with ACS, but without OSA (AHI <15/h) were included as a reference group. The primary endpoint of the study was the development of a composite of cardiovascular events (cardiovascular death or acute MI, stroke, hospital admission due to heart failure, unstable angina or TIA). After a follow-up of 3.35 years, the incidence of cardiovascular events was similar in the two groups (CPAP: 16%; usual care: 17%; HR 0.89; 95% CI [0.68–1.17]). The median adherence to CPAP treatment was 2.78 h/night. No differences were found in the incidence of cardiovascular events in the usual care group versus the reference group without OSA (17% and 15%, respectively). Nor was there an association between the number of cardiovascular events and the hours of CPAP adherence or the severity of OSA. CPAP treatment was associated with a modest improvement in daytime sleepiness and blood pressure, but not with any improvement in quality of life.
The fundamental difference between the SAVE and the ISAACC studies concerns the samples used. While the SAVE study included patients with chronic coronary or cerebrovascular disease, the patients in the ISAACC study were in the acute phase of CHD.53,54 Both studies found that treatment with CPAP did not reduce cardiovascular risk in patients with asymptomatic OSA, and highlight the lack of any significant effect of CPAP in secondary cardiovascular prevention.53,54 Two recent meta-analyses also confirm that CPAP does not improve survival or prevent the development of major cardiovascular events in patients with OSA.55,56 There may be several explanations for this.
First, poor adherence to CPAP treatment is an important limitation in all studies. The average use is less than 4 h/night, due at least in part to the patients’ low level of symptoms (patients with a high level of symptoms were not included for ethical reasons). Perhaps better results would have been recorded if patients with more symptoms had been included, given that excessive daytime sleepiness is a sign of disease severity. For example, the effect of CPAP on blood pressure is greater in patients with more symptoms. However, at present, we cannot claim that better adherence would have produced different results. Factors influencing adherence to CPAP therapy need to be identified, and the issue of whether better adherence improves cardiovascular endpoints needs to be investigated in greater depth.57
Second, the reference procedure for the diagnosis of OSA is polysomnography. AHI is the score used to determine severity, but it may not fully reflect the complexity of the disease. It is common to see patients with low AHI with AHT and high levels of symptoms, and it is also quite common to see patients with AHI >30/h and very low levels of symptoms. AHI does not take into account important aspects of respiratory events such as the magnitude of the associated desaturation or the presence of awakenings. This means that it is likely to include very heterogeneous patients with different disease phenotypes, a circumstance that may explain the negative results. The use of new diagnostic and/or prognostic criteria in patients with OSA that allow better risk stratification, as well as the use of biomarkers able to identify subgroups of patients with OSA with a high risk of cardiovascular or metabolic events, might facilitate reassessment of the role and efficacy of OSA treatment in both primary and secondary prevention.58
Third, the treatment of OSA with CPAP may not be effective in reducing recurrent cardiovascular events in patients with advanced or symptomatic atherosclerotic vascular disease, such as the patients included in the SAVE and ISAACC studies. Reversing or stabilising an altered vascular structure is more difficult than preventing its initial alteration, therefore the results of these studies do not rule out the possible effect of OSA treatment on primary prevention.57 Moreover, the ischaemic preconditioning generated by the nocturnal cycles of hypoxia–reoxygenation has been considered as one of the causes of the decrease in cardiovascular and cerebrovascular mortality in elderly patients with OSA and may be involved in some way in this apparent lack of efficacy of CPAP in secondary prevention.59
Fourth, it has recently been suggested that the design and development of clinical trials that analyse the hypothetical efficacy of OSA treatment in cardiovascular prevention need to be modified.60 The changes proposed include the modification of the inclusion criteria for patients with OSA to recruit patients with greater severity of disease; the improvement of the diagnostic criteria for asymptomatic OSA; and the inclusion of criteria for arterial oxygen desaturation, bearing in mind that the hypoxaemic burden is considered the main mediator in the increased risk of morbidity and mortality in patients with OSA. The identification and exclusion of patients with OSA with low adherence to CPAP treatment has also been suggested, as has the use of alternative (or complementary) statistical techniques to the intention-to-treat analysis, although this might underestimate the hypothetical efficacy of CPAP by including patients with low adherence.
CPAP can reduce various inflammatory biomarkers involved in the onset, progression and instability of atherosclerotic cardiovascular disease and which are elevated in patients with OSA, such as C-reactive protein, tumour necrosis factor alpha, interleukin (IL) 6, IL-8, intercellular adhesion molecule, vascular cell adhesion molecule and selectins.61 In spite of this, and although it may cause some degree of haemodynamic improvement (by increasing left ventricular ejection fraction) and may even prevent arrhythmias, in clinical studies these potential effects have not been converted into a significant reduction in recurrent cardiovascular events in patients with established cardiovascular disease. More studies are needed to establish the true role of CPAP treatment in patients with OSA in both primary and secondary prevention.
Clinical Recommendations
Patients who present two of the three cardinal criteria of OSA (snoring, apnoea and/or daytime sleepiness or tiredness) should be referred to the sleep disorders unit and OSA should also be considered in the differential diagnosis of bradyarrhythmia.62 Although the clinical utility of CPAP in secondary cardiovascular prevention has not been demonstrated, the SAVE study recorded an improvement in daytime sleepiness and a reduction in days off work, even in patients initially considered asymptomatic.52 For this reason, physicians should ask specifically about the presence of symptoms suggestive of OSA as part of the routine clinical assessment of patients with cardiovascular pathology.
Conclusion
Epidemiological and clinical evidence indicates that OSA may be a potentially modifiable risk factor for arterial vascular disease. OSA has been associated with a higher incidence of hypertension and cardiovascular disease. However, clinical trials on the efficacy of CPAP in primary and secondary cardiovascular prevention have not demonstrated a significant reduction in the incidence and/or recurrence of cardiovascular events. A number of measures are now needed to shed more light on the relationship between OSA, treatment with CPAP and vascular risk: the use of new diagnostic and/or prognostic criteria to improve the clinical stratification of patients with OSA; the identification of variables associated with better adherence to CPAP treatment; the development of new therapeutic techniques for patients with OSA; the use of biomarkers that identify subgroups of patients with OSA with greater vascular or metabolic risk and make it possible to intervene in subclinical phases of atherosclerotic disease; and the modification of the design and development of clinical trials that analyse cardiovascular risk in patients with OSA treated with CPAP.