Hypertension is a major independent risk factor for cardiovascular (CV) diseases, including cardiac death, coronary heart disease, heart failure, stroke and chronic kidney disease. Therefore, early diagnosis, prevention and optimal management of hypertension is essential.1 Hypertension poses a growing public health burden: the number of adults with elevated blood pressure (BP) increased from 594 million in 1975 to 1.13 billion in 2015, and it is estimated that the number of people with hypertension will be close to 1.5 billion by 2025.2,3 During the past decades, BP measurement has evolved from manual measuring to fully automatic monitoring; ambulatory BP monitoring (ABPM) was first described in 1964.4 Since then, the importance of nocturnal BP has been recognised.5 This article aims to understand the clinical significance, therapeutic implications and optimal measurement of nocturnal hypertension.
Nocturnal Hypertension
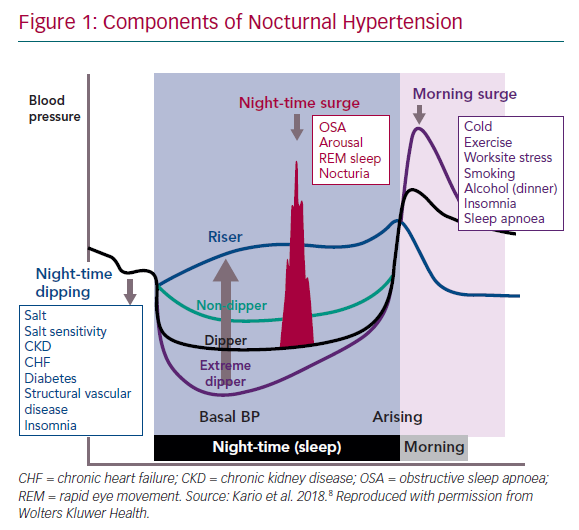
BP variation over 24 hours normally follows a pattern, with a peak in the early morning hours and a decrease at night, known as dipping. This variation is the result of an endogenous circadian rhythm, which exerts its peak at around 9 pm, and behavioural effects that are superimposed on the circadian pattern, causing much of the observed day-night pattern in BP.6 In general, BP starts declining from late evening onwards, reaches a nadir around midnight and rises just after awakening in the morning (Figure 1).7,8 People are defined as dippers if their nocturnal BP falls by >10 % of the daytime average BP value; however, dipping status varies from day to day and the classification of patients into dippers and non-dippers is not reproducible over time.1,5,9 Non-dipping status is associated with sleep disturbance, obstructive sleep apnoea (OSA), obesity, high salt intake in salt-sensitive people, orthostatic hypotension, autonomic dysfunction, chronic kidney disease, diabetic neuropathy and older age.10–16
In recent years, it has been recognised that nocturnal BP is, in general, a better predictor of the risk of fatal and nonfatal CV events (stroke, MI and CV death) and organ damage than daytime BP in hypertensive and renal transplant patients.10,15–19
Night-to-day ratio is also a significant predictor of CV events, and patients defined as non-dippers have an increased CV risk.15 A non-dipping BP pattern has been shown to be predictive of a range of CV events, including total CV death, sudden death, nonfatal CV events and nonfatal stroke, in people with and without carotid atherosclerosis.20 In patients with heart failure with preserved ejection fraction, a non-dipping pattern was found to be an independent risk factor for future CV events, including recurrence of hospitalisation for heart failure and cognitive dysfunction.21,22 People who experience a night-time BP decrease of ≥20% are termed extreme dippers; this may be associated with an increased risk of ischaemic stroke and silent cerebral diseases.23 An exaggerated morning surge in BP is also associated with an increased risk of stroke.24 Therefore, nocturnal hypertension has become an important therapeutic target for the prevention of CV events in patients with hypertension.
Obstructive Sleep Apnoea in Patients with Hypertension
OSA is a sleep disorder characterised by frequent episodes of partial or complete collapse of the upper airway during sleep.25 A large body of evidence supports the association of OSA with hypertension, CV disease and disorders of glucose metabolism.26,27 The relationship between OSA and hypertension is bidirectional: not only does OSA predispose patients to develop hypertension, but there is also a greater incidence of OSA in hypertensive patients.28–30 Hypertension in patients with OSA is predominantly nocturnal, and non-dipping BP is common in patients with OSA.14,31–33 The average peak and the maximal nocturnal BP values may be related to the nocturnal BP surge triggered by the apnoea or hypopnea episodes of OSA.34
The prompt diagnosis and treatment of OSA are essential to the management of hypertensive patients, since OSA is a common cause of resistant hypertension.35 Furthermore, the constant sleep fragmentation associated with OSA and related conditions, such as OSA–hypopnoea syndrome is associated with disrupted and short sleep, excessive daytime sleepiness, fatigue, headaches and high rates of morbidity and mortality.36 Poor quality and quantity of sleep has a substantial negative impact on CV health and all-cause mortality, type 2 diabetes, hypertension, respiratory disorders and obesity at all ages.34–38
Importantly, OSA can be treated; continuous positive airway pressure (CPAP), the first-line therapy for moderate to severe OSA syndrome, reduces the 24-hour mean BP by approximately 2 mmHg.39 Active or supportive measures to increase quality or quantity of sleep may also be beneficial to patients with OSA–hypopnoea syndrome.39
Challenges of Diagnosing Obstructive Sleep Apnoea and Related Hypertension
OSA and nocturnal hypertension are often not recognised, or masked.40 Unlike white coat hypertension, in which BP is elevated in clinical settings but normal at other times, people with masked hypertension have normal BP in the office, but elevated BP at other times.1 Masked hypertension has been reported in approximately 15% of patients with a normal BP in the office. It is associated with dyslipidaemia, increases in arterial stiffness, raised plasma B-type natriuretic peptide level and urinary albumin:excretion ratio, increased risk of developing diabetes, and sustained hypertension and CV disease.1,41,42 Masked nocturnal hypertension has been associated with markers of CV risk, even in hypertensive patients with well-controlled self-measured home BP.43–45 Therefore, it is important that a uniform, reproducible method for the measurement of nocturnal BP is established. Nocturnal BP is generally considered the average of BP readings recorded during the period most likely coinciding with sleep time.46 Night-time is usually set a priori (e.g. from 10 pm to 6 am), without considering variations in sleeping times between patients and individual patient fluctuations. Furthermore, the current methods are unable to differentiate between time in bed and time asleep. Issues such as differing methods for monitoring nocturnal BP, different definitions of nocturnal time, diagnostic thresholds and opinions of whether to control abnormal nocturnal BP, have hindered progress in the appropriate management of patients with hypertension.47
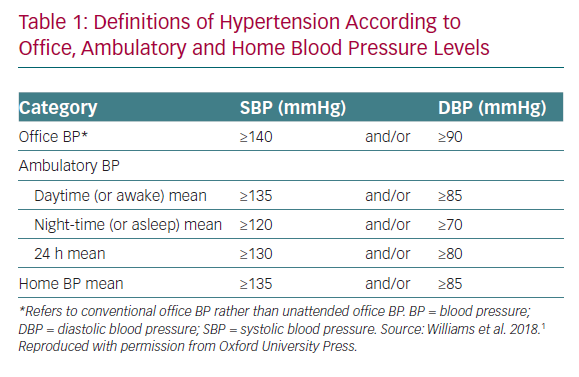
Ambulatory BP monitoring (ABPM) has historically been the gold standard for measuring nocturnal BP. It provides the average of BP readings over a defined period, usually 24 hours. The device is programmed to record BP at 15–30 minute intervals (often 60 minute intervals at night), and calculates average BP values for daytime, night-time and over 24 hours.1,48 According to the latest European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) guidelines, the thresholds for diagnosis of hypertension based on ABPM are ≥130/80 mmHg over 24 hours, ≥135/85 mmHg in the daytime and ≥120/70 mmHg at night (Table 1).1 The night-time BP is calculated as the average of night-time measurements, which is typically set between 10 pm and 6 am, so that average readings often do not reflect BP at the time of sleep. It is well established that ABPM is more sensitive than office BP in predicting CV events.1,49 The use of ABPM also enables the diagnosis of white coat and masked hypertension.1
Despite the advantages of ABPM, its availability is limited, and the devices are relatively expensive.48,50 As a result, not every patient with hypertension will have ABPM measurements and nocturnal hypertension can remain undetected. Furthermore, ABPM devices are cumbersome and often uncomfortable, and can cause sleep disturbance because of the device cuff inflation and frequency of measurements, resulting in a measured nocturnal BP value that is different from the true BP when the patient has an undisturbed sleep.51 A prospective study found that night-time BP rises and loses its prognostic significance in the patients who perceive a sleep deprivation by at least 2 hours during overnight monitoring.52 Finally, the definitions of masked and white-coat uncontrolled hypertension defined with ABPM show poor reproducibility over time, reducing its long-term prognostic value.53 Therefore, there are several reasons to believe that there are still unmet needs for obtaining high-quality BP values during sleep.
The Role of Home Blood Pressure Monitoring in the Diagnosis and Treatment of Hypertension
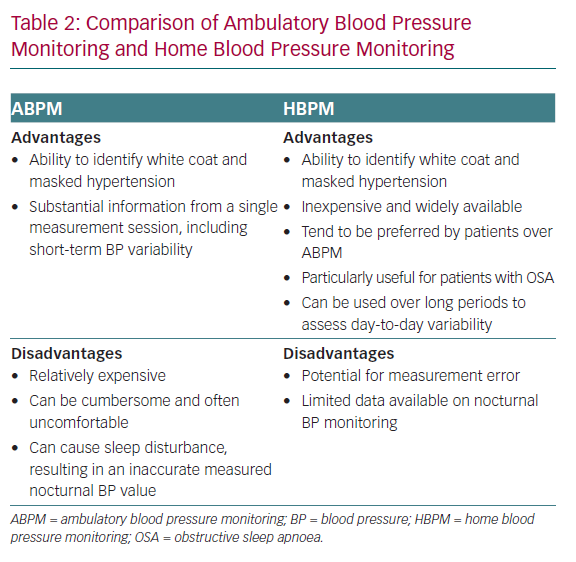
The use of home BP monitoring (HBPM) is widespread, well accepted and is relatively inexpensive compared with ABPM.1 A comparison of the advantages and disadvantages of the two techniques is given in Table 2. Patient preference might influence adherence to BP-lowering medication. A 2014 study of 119 patients found that patients with hypertension prefer HBPM to ABPM.54 This is an important finding and warrants further investigation. Early studies suggested that the use of HBPM could improve adherence and the level of BP control to target.55 A recent trial confirmed that the use of connected HBPM devices improves the percentage of patients that achieve target values and the time in which the target is reached.56 Therefore, home BP monitoring has become widely recommended for the management of hypertension, as it enables the collection of more data away from the office setting and in a patient’s normal environment.1
The ESC/ESH guidelines state that the lack of nocturnal readings in HBPM is a disadvantage, and that home nocturnal BP monitoring is not available.1 However, recent technological advances in self-monitoring devices have facilitated the development of several kinds of home measurement devices for nocturnal BP, and a number of studies have shown that nocturnal HBPM is both feasible and effective.8,57–65 The first data on nocturnal BP measurement were obtained in 2001, when a Japanese research group examined the impact of a single nocturnal BP measurement on sleep quality.63 The same research group later found that the reproducibility of a single nocturnal BP measurement as assessed using an HBPM device was not good.61 Subsequently, HBPM devices have produced data that correlate more closely with ABPM measurements.64
A number of nocturnal HBPM devices have been validated. These include two oscillometric upper-arm cuff home BP monitors (Omron HEM-747IC-N; OMRON Healthcare and the HEM-5001; Medinote, Omron Healthcare).66,67At present, no comparative studies have been performed for these devices.
In order to fully assess the benefits of nocturnal HBPM, studies are needed to assess its reproducibility within subjects and the prognostic ability. In a pilot study, 39 patients with OSA underwent polysomnography, clinic BP measurements, and HBPM using a device that allowed daytime (3 days, two duplicate readings per day) and automated night-time BP measurement (3 nights, three readings per night).65 HBPM showed a stronger correlation than ABPM to the severity of OSA.60 Another study (n=30) evaluating nocturnal BP of treatment-naive individuals using an HBPM device found that night-time BP measurements were significantly lower in subjects without left ventricular hypertrophy (LVH) compared with those with LVH, while daytime readings were not significantly different between the two groups.62 A recent meta-analysis found that the nocturnal HBPM and ABPM measurements provided similar values (HBPM readings were 1.4 mmHg higher for systolic and 0.2 mmHg lower for diastolic values) compared with ABPM measurements, and both were similarly associated with measures of target organ damage (left ventricular mass index).58
The NightView HBPM Device
To date, all studies on nocturnal HBPM have been small. Practical issues need to be addressed, including the optimal number of nocturnal BP readings needed to obtain clinically meaningful information. The most accurate assessment of nocturnal BP would require frequent measurements during sleep, but this may cause sleep interruption and would be expensive for individuals with the current method of ABPM. On the other hand, HBPM with cheaper and validated available devices is not suitable for night-time BP measurements because these need patients to initiate the readings. Therefore, a reasonable compromise is needed. Available data suggest that HBPM may prove to be more feasible, affordable and widely available for routine continued clinical assessment of nocturnal BP, but a standardised validated method for the measurement of nocturnal BP is needed.
NightView (OMRON Healthcare, HEM9601T-E3) is a connected wrist type HBPM device for use in adults with wrist circumference ranging from 13.5 cm to 21.5 cm (Figure 2). In addition to normal daytime measurements, it also automatically takes BP measurements during sleep. The NightView nocturnal mode is selected when the patient goes to bed and automatically takes three measurements whether the user is asleep or awake at the time of measurement: 4 hours after going to bed, at 2 am and 4 am. These measurements are silent and do not cause sleep disturbance. If a nocturnal measurement fails because of an error such as movement error, the monitor will try to take another measurement a minute later (it tries only once).
The device is connected via Bluetooth and data management is done via the Omron Connect app. NightView has been clinically validated in sitting and in supine position (with palm placed sideways, upwards and downwards), according to the ANSI/AAMI/ISO81060-2:201 protocol and has passed all criteria.68,69 A comparison with ABPM in 85 subjects found similar results in office and out of office.68,69 This improved possibility to detect masked nocturnal hypertension will allow optimisation of BP-lowering medication, with the ultimate goal of optimising the achievement of BP target values and reduce CV events.
Treatment of Nocturnal Hypertension
The impact of controlling night-time BP is potentially greater in people with hypertension who are taking medication than in those who are not taking medication, because the BP-lowering effect of a once-daily BP drug may not persist for 24 hours even in patients with well-controlled daytime BP.8 Therefore, it has been suggested that taking BP-lowering medication at night may be more effective than in the morning.
The Ambulatory Blood Pressure Monitoring and Cardiovascular Events (MAPEC) study, which included 2,156 hypertensive adults with a median follow-up of 5.6 years, found that the administration of at least one BP-lowering medication at bedtime was more effective than morning dosing, both in terms of lowering nocturnal BP and restoring the expected variability in BP, and for reducing CV events and total mortality.70
Recently, the Hygia Chronotherapy Trial in Spain involved 19,084 patients in the primary care setting.71 The trial was designed to compare treatment with BP-lowering medications at bedtime with usual upon awakening hypertension therapy in terms of CV risk reduction. The results have sparked much interest and debate, since they suggest that patients who took medication at bedtime, compared with upon awakening, had more than half the rate of CV events during the mean 6.3 year follow-up, attributable only to a 3.3/1.6 mmHg greater fall in night-time BP and no difference in daytime BP.71 However, the study has a number of important limitations. The two groups differed significantly: there were more patients with previous CV events and more patients taking diuretics and beta-blockers in the awakening medication group than in the group receiving bedtime medication.72 The prevalence of side-effects and non-adherence was unexpectedly similar between groups, and the relative risk reduction recorded was unexpectedly high for the reported small differences in BP recorded at night.73 Doubts concerning ethics, randomisation and plausibility have led to questioning the credibility of the results.73,74 Further well-designed randomised controlled trials are needed to provide evidence that treating nocturnal BP is beneficial.
Conclusion
Nocturnal hypertension, as assessed by ABPM, is a stronger predictor of CV events than daytime hypertension, suggesting that monitoring nocturnal BP is an important method of stratifying CV risk due to high BP, although the additional CV benefits of lowering night-time BP is still debated. Furthermore, there is a need for the prompt diagnosis and treatment of hypertension and OSA to help address the increasing CV morbidity and mortality due to the frequent co-morbidity. A recent review found that certain antihypertensive medications are more effective than others in regulation of nocturnal BP, but that the timing of drug administration is key to the reduction of night-time BP. However, the authors concluded that further studies are needed.75,76
Night-time ABPM may interfere with sleep quality because of the intrusive cuff inflation and the frequency of measurements, often resulting in disturbed sleep and inaccurate nocturnal BP measurements. The use of nocturnal HBPM is potentially a reliable, cheaper and practical alternative to ABPM for the repeated measurement of BP during sleep. The use of HBPM devices is widespread, well accepted by users and is relatively inexpensive, it may prove to be more feasible and widely available for routine clinical assessment of nocturnal BP, reducing primary care consultations and healthcare costs. Preliminary data suggest that nocturnal HBPM is feasible and provides prognostic information similar to that of ABPM. The NightView HBPM device offers the possibility of daytime and night-time BP measurements with minimal sleep disturbance.
Further data are needed on the prognostic significance of nocturnal BP measured by HBPM in terms of CV outcomes, optimal schedules for measurement, and the value of administering BP medications at different time of the day.