Hypercholesterolemia has been known for several years to be a major risk factor in the development of atherosclerosis and consecutively cardiovascular disease. This epidemiological concept has been widely confirmed using different strategies that have reduced low-density lipoprotein (LDL) levels and cardiovascular events (morbidity and mortality) in primary as well as in secondary prevention, in different groups of patients.
Because statins have broadly demonstrated a significant improvement in prognosis for reducing atherosclerotic heart disease, they are considered the treatment of choice for reducing LDL.1,2 However, some patients present secondary effects (mainly muscular pain or hepatic enzyme elevation), which can lead to non-adherence or even discontinuation of their therapeutic regimen. Statins may also exert diabetogenesis.3 In addition, although statins are effective lipid-lowering drugs, there remains a residual cardiovascular risk in many patients. In this context, ezetimibe was developed as an alternative drug for statin-intolerant patients or as an addition to statins. Ezetimibe may further improve the prognosis of atherosclerotic patients.4 However, even in combined statin and ezetimibe therapy, some patients still present elevated (“non-optimal”) LDL levels, cardiovascular risk and events. Thus, there was a need to identity additional effective and safe lipid-lowering strategies to be used either in combination with statins, ezetimibe or alone.
Proprotein convertase subtilisin kexin type 9 (PCSK9) is a liver secretory enzyme that regulates plasma low-density lipoprotein (LDL) cholesterol (LDL-C) levels through modulation of LDL receptor (LDLR) density on hepatocyte surface. Monoclonal antibodies targeting PCSK9 provoke significant lowering of LDL-C, non-high-density lipoprotein cholesterol and lipoprotein(a) as monotherapy or in combination with other lipid-lowering drugs. In fact, only two PCSK9 inhibitors are clinically available: alirocumab and evolocumab. Interestingly, PCSK9 inhibitors demonstrate favourable impact on atherosclerotic plaque progression and lipidic profile of patients with and without familial hypercholesterolemia.5–7 Importantly, the available data suggest that PCSK9 inhibitors do not adversely affect glucose metabolism, do not increase the incidence of new onset diabetes mellitus3 and reduce cardiovascular events in patients with type 2 diabetes mellitus at least as much as in those without. However, although there is a possible association of PCSK9 levels with kidney function, no data are available for the administration of PCSK9 inhibitors in patients with severe chronic kidney disease.8 In addition, the physiological limits of lowering LDL-C are not yet established.9
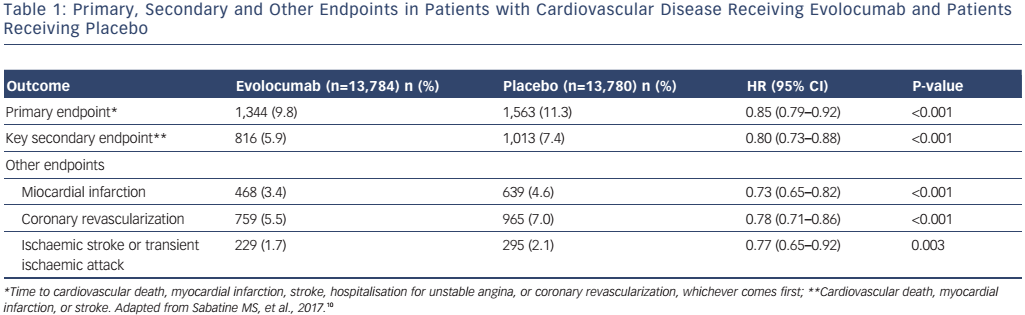
The most interesting and important analysis end-point of PCSK9 inhibitor treatment is related to the long-term outcomes of broad treatment populations with evolocumab (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk [FOURIER])10 and alirocumab (ODYSSEY).11 Some trials are on-going (ODYSSEY) while others have been completed and their results are available.10 Previous long-term studies using evolocumab, including the Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV) (78 weeks), Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER-1) (4 years), and pooled analyses of the Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK9 in Different Populations (PROFICIO) (~1 year) have shown sustained LDL-C reduction, without evidence of safety concerns, regardless of LDL-C level achieved.6,7,12 Importantly, the recently presented FOURIER trial analyzed the outcome of 27,564 patients with atherosclerotic cardiovascular disease and LDL-C >70 mg/dl who were receiving statin (±ezetimibe) therapy and were randomly assigned to receive evolocumab (either 140 mg every 2 weeks or 420 mg monthly) or matching placebo as subcutaneous injections.10 The median pre-randomization LDL-C was 92 mg/dl in both cohorts of patients. With evolocumab, LDL-C was quickly (in approximately 4 weeks) and significantly reduced, and thereafter sustained (median: 30 mg/dl). Furthermore, 42 % of patients treated with evolocumab achieved levels ≤25 mg/dl (versus <0.1 % in the placebo group). The median duration of follow-up was 2.2 years. The primary endpoint (composite of cardiovascular death, myocardial infarction [MI], stroke, hospitalization for unstable angina or coronary revascularization) was reduced with evolocumab compared to placebo (1,344 patients [9.8 %] versus 1,563 patients [11.3 %]; HR 0.85; 95 % CI [0.79–0.92]; p<0.001). These results were mainly driven by reductions in MI, stroke, and coronary revascularization (see Table 1). Also, the secondary endpoint (composite of cardiovascular death, MI or stroke) was significantly improved by evolocumab (see Table 1) but without safety concerns. Adverse events in the evolocumab (n=13,769) and placebo (n=13,756) groups included injection-site reaction, allergic reaction, muscle-related event, rhabdomyolysis, cataract, adjudicated case of diabetes, and neurocognitive event; however, there were no significant differences in major side-effects or laboratory results (aminotransferase >3 x ULN and creatinine kinase >5 x ULN) between the two groups. Importantly, the incidence of neurocognitive events, cataracts and new-onset diabetes were similar between the two treatment arms. Only injection site reactions were more frequent with evolocumab (82.1 % versus 1.6 %). Post-baseline anti-evolocumab antibodies were detected in 0.3 % patients, with no detected neutralizing antibodies.
The key findings of the FOURIER trial are as follows:
- Treatment with evolocumab in patients with established cardiovascular disease, who were receiving high to moderate intensity statin therapy, over a median of 2.2 years compared with placebo resulted in significant and maintained lowering of LDL-C and significant cardiovascular risk reduction.
- Levels of even LDL-C of 22 mg/dl had beneficial effects (reduction of the key secondary endpoint) without major safety concerns. Furthermore, the Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects (EBBINGHAUS) cognitive function trial13 conducted in FOURIER patients showed non-inferiority of evolocumab in comparison to placebo (data presented at the American College of Cardiology Congress 2017, not yet published).
For alirocumab, the other clinically available PCSK9 inhibitor, a large Phase III program (ODYSSEY) has been conducted with 12 fulfilled trials designed to demonstrate superiority of alirocumab versus placebo, the superiority of the addition of alirocumab over up-titration of statin and/or ezetimibe.5 In addition, a large Phase III study (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab [ODYSSEY OUTCOMES]) is on-going to evaluate the long-term effect of alirocumab on cardiovascular events in approximately 18,000 patients after an acute coronary syndrome on a background of intensive statin therapy.11 The important differences between the FOURIER and ODYSSEY OUTCOMES trials are:10,11
- ODYSSEY OUTCOMES includes a post-acute coronary syndrome population versus established atherosclerotic coronary disease in FOURIER.
- Two different doses of alirocumab, which allows some flexibility in titrating with regard to the LDL-C level, are used in ODYSSEY OUTCOMES versus evolocumab, either 140 mg every 2 weeks or 420 mg every month, according to patient preference in FOURIER.
- The primary composite outcome in FOURIER includes cardiovascular death, MI, stroke, hospitalization for unstable angina or coronary revascularization, but ODYSSEY OUTCOMES does not include revascularization in its key primary composite outcome.
- FOURIER includes a larger population (n=27,564) than ODYSSEY OUTCOMES (approximately 18,000), but with shorter follow-up (approximately 2 years versus 3 years).
Thus, both trials are complementary and aimed at demonstrating that PCSK9 inhibition reduces cardiovascular risk via potent LDL-C reduction and that this approach is safe in the long term. However, the longest follow-up of these trials is approximately 3 years. Therefore, these trials and future data will provide a sense of the true cardiovascular benefit and a more accurate understanding of very long-term (e.g. >10 years) treatment risks and possible physiological effects of potent (e.g. LDL <40 mg/dl) lowering of LDL-C.







