Heart failure (HF) is a growing epidemic, with an estimated 6.2 million adults (≥20 years) affected between 2013 and 2016 in the US.1 The prevalence is increasing and is expected to reach 8 million by 2030.1 Mitral regurgitation (MR) is common in HF patients, especially in those with ischaemia, left ventricular (LV) dysfunction or dilated cardiomyopathy. MR in the absence of structural abnormalities of the mitral valve complex is called functional or secondary MR (SMR) as the problem lies with the ventricle. SMR is the most common form of MR and in 2011 its prevalence was reported to be 16,250 per 1 million in the US population – a total of 5.2 million people.2
It creates a vicious cycle of worsening HF and SMR – volume overload and dilated left ventricle (from HF) results in a dilated mitral annulus and tethered mitral leaflets (severe SMR), which in turn causes more HF. This cycle can be interrupted in several ways:
- Medical therapy.
- Transcatheter mitral valve repair with MitraClip (Abbott).
- Heart transplantation or LV assist device (LVAD).
- Surgical intervention.
In a meta-analysis of 53 studies and 45,900 patients, the presence of SMR was associated with an increased risk of cardiac mortality, HF, hospitalisations, transplant or death.3 In this article, we discuss the aetiology, diagnosis and management of SMR in chronic HF.
Aetiology of Secondary Mitral Regurgitation
There can be multiple aetiologies of SMR in HF. They are usually related to the aetiology of HF, thus establishing HF as the primary basis of the disease. The aetiology of SMR includes:
- Tethered leaflet(s) due to ischaemic cardiomyopathy: MI and ischaemic cardiomyopathy can lead to regional wall motion abnormalities, most commonly resulting in posterior leaflet tethering and anterior leaflet override.
- Dilated annulus secondary to severe LV dysfunction and long-term LV ischaemic cardiomyopathy causing non-coaptation of mitral leaflets.
- Dilated annulus from dilated non-ischaemic cardiomyopathy with a similar mechanism as the previous aetiology.
- Papillary muscle dysfunction related to acute or chronic ischaemia secondary to coronary artery disease.
- Dilated left atrium where severe enlargement of the left atrium can result in a dilated mitral annulus and SMR.
Diagnosis of Secondary Mitral Regurgitation
The initial diagnosis of MR may be based on a physical examination. However, the systolic murmur can be low-pitched and may be missed in cases of SMR. Echocardiography is the gold standard for the diagnosis of MR. It also helps to quantify the degree of MR based on established criteria. According to the American Society of Echocardiography, severe MR is consistent with: vena contracta width ≥0.7 cm; effective regurgitant orifice area (EROA) ≥0.4 cm2; regurgitant volume (RVol) ≥60 ml; and regurgitant fraction (RF) ≥50%.4
The severity of MR is classified in grades as mild (1+), moderate (2+), moderate-severe (3+) or severe (4+). Moderate-severe or 3+ MR is defined by an EROA of 0.30–0.39 cm2, RVol of 45–59 ml and RF 40–49%. The measurement of these parameters is difficult and requires experience and time. It can also be limited by measurement errors related to the patient’s body size and build. If there is discrepancy between the echocardiographic results and the patient’s presentation and physical exam, other modalities such as transoesophageal echocardiogram, cardiac MRI or left ventriculography can be used to determine SMR severity.
Management of Secondary Mitral Regurgitation
Medical therapy
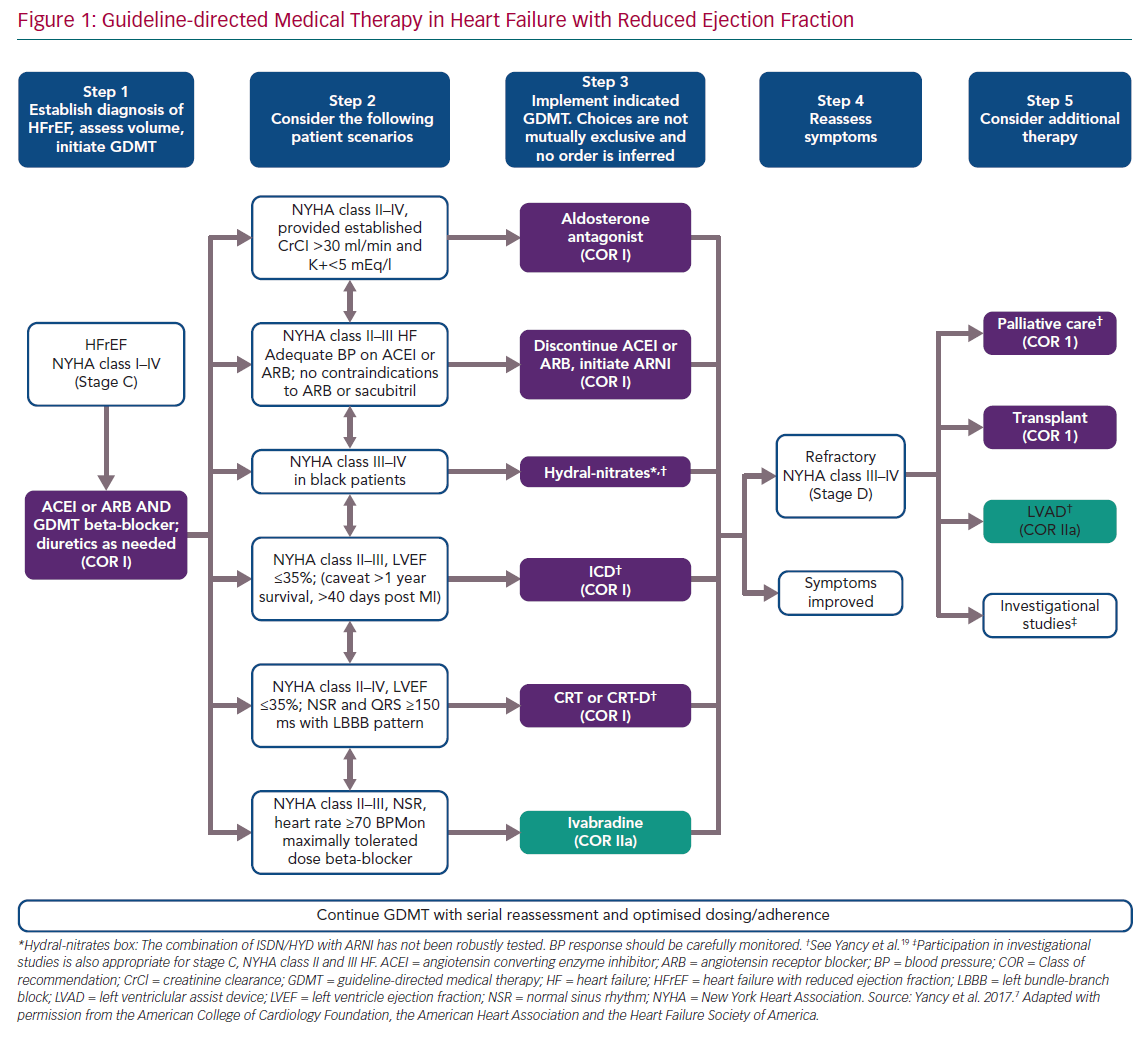
Medical therapy is the mainstay of treatment in patients with HF with reduced ejection fraction (HFrEF) including those with SMR. In a recent study of 163 consecutive patients with HFrEF (left ventricular ejection fraction [LVEF] <40%) and grade 3–4+ SMR who received maximally tolerated guideline-directed medical therapy (GDMT), SMR was assessed at baseline and after a median follow-up period of 50 months. Of the 31% (n=50) of the total group who had severe SMR at baseline, 38% improved to non-severe SMR (≤2+), while 18% of the non-severe participants progressed to severe SMR. Severe SMR, whether it was sustained or developed from non-severe SMR, was the most important independent prognostic determinant of major adverse cardiac events (defined as a composite of all-cause death and the need for heart transplantation or hospitalisation for HF and/or malignant arrhythmias), with an adjusted OR 2.5 (95% CI [1.5–4.3], major adverse cardiac events 83% versus 43%).5 In a sub-analysis of the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional MR (COAPT) trial involving the 614 participants who presented with 3+ or 4+ MR at the start of the study, a reduction in SMR to <2+ was seen in 34% of those who were given GDMT.6 According to the American Heart Association (AHA)/American College of Cardiology (ACC) valve guidelines, GDMT is the first-line therapy for HFrEF and SMR (Figure 1) and the only Class I indication for treatment of SMR.7 GDMT includes:
- beta-blockers to reduce risk of HF hospitalisations and mortality;
- angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) to reduce risk of HF hospitalisations and mortality;
- angiotensin-receptor-neprilysin inhibitors (ARNI) to replace ACEI or ARB to reduce risk of HF hospitalisations and mortality;
- mineralocorticoid receptor antagonists for mortality reduction;
- hydralazine-nitrates in African-Americans for mortality reduction;
- ivabradine to reduce risk of HF hospitalisations; and
- diuretics to reduce congestion.
In addition to pharmacological management, cardiac resynchronisation therapy (CRT) should be considered in patients with New York Heart Association (NYHA) Class II–IV HF, LVEF ≤35%, normal sinus rhythm and left bundle branch block with QRS >150 ms. In these patients, CRT can also facilitate LV reverse remodelling and reduce associated SMR.8 Early involvement of HF teams is essential for quick optimisation of therapy in patients with SMR and HF. If medical therapy fails, other interventions can be considered depending on the patient’s condition.
Surgical Intervention
Surgical intervention for SMR has been tested in randomised controlled trials (RCTs) by the Cardiothoracic Surgical Trials Network (CTSN). The first CTSN trial compared mitral valve repair versus replacement in 251 patients with severe ischaemic SMR. The investigators reported that mitral valve repair was associated with a significantly higher rate of recurrent moderate or severe MR (58%) as compared with mitral valve replacement (3.8%) at 2 years, resulting in more HF and cardiovascular hospitalisations. The LV end-systolic volume index (LVESVI) or LVEF were not significantly different during follow-up. Similarly, there was no significant difference in rates of all-cause mortality and major adverse cardiac or cerebrovascular events (MACCE) between repair and replacement groups.9
The second CTSN trial compared coronary artery bypass surgery (CABG) alone with CABG plus mitral valve repair in 301 patients with coronary artery disease and moderate SMR. The LVESVI and LVEF were similar in the two groups at 2-year follow-up. Moderate or severe MR was significantly higher in the CABG alone group (32.3%) compared with CABG plus mitral repair (11.2%). All-cause mortality, MACCE or rates of readmission and cardiovascular readmission were similar between the groups. The combined group had a higher rate of early neurological events, probably related to longer cross-clamp times and supraventricular arrhythmias.10 The AHA/ACC valve guidelines give a Class IIb indication for mitral valve surgery in patients with isolated severely symptomatic moderate-severe or severe SMR.11 However, none of these trials compared cardiac surgery to GDMT and these trials did not involve optimisation of medical therapy by HF specialists. Therefore, the role of cardiac surgery in isolated SMR remains uncertain. In patients undergoing CABG or another cardiac surgery, mitral valve surgery is considered reasonable for SMR (US guidelines – Class IIa; European guidelines – Class I [LVEF >30%] and Class IIa [LVEF ≤30%]).11,12
Transcatheter Mitral Valve Repair with MitraClip
Transcatheter edge-to-edge repair of the mitral valve with MitraClip has emerged as a catheter-based intervention for the treatment of SMR. This was recently evaluated in two clinical trials, Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA-FR) and COAPT, with different results.
MITRA-FR Trial
The MITRA-FR trial was a multicentre, randomised, open-label clinical trial of transcatheter mitral valve repair with MitraClip versus medical therapy in symptomatic patients with SMR conducted in France.13 Key inclusion criteria were: severe MR defined as an EROA >20 mm2 or RVol >30 ml/beat; ejection fraction (EF) 15–40%, at least one HF hospitalisation in the last year and patients were ineligible for surgery. Of the 452 screened patients, 304 patients were randomised 1:1 to MitraClip versus medical therapy. The mean age of the participants was 70 years, 60% had ischaemic cardiomyopathy, two-thirds had NYHA III–IV HF at baseline. The mean LVEF was 33%, mean EROA was 0.31 cm2, mean LV end-diastolic volume (LVEDV) index was 135 ml/m2 and mean regurgitant volume was 45 ml.
Medical therapy was optimised by the local investigators. The majority of the patients were on loop diuretics, beta-blockers and ACEI/ARB/ARNI. CRT was present in 30% of the patients in the MitraClip arm compared with 23% of the control arm. Of the 152 patients in the MitraClip arm, 14 patients did not undergo MitraClip implantation. The reasons for this included: operator unable to grasp the mitral valve leaflets (n=3), cardiac tamponade (n=2), cardiogenic shock (n=1), and ‘not attempted’ due to other reasons (n=8). Technical success was achieved in 96% of the patients with MitraClip implantation. At discharge, 91.9% of these patients had MR grade <2+.
The primary endpoint of all-cause mortality or unplanned HF hospitalisation was similar in the MitraClip (54.6%) and control arms (51.3%; OR 1.16; 95% CI [0.73–1.84]). The results were similar in most of the major sub-groups such as LVEF <30% or >30%, EROA of <0.3 cm2, 0.3–0.4 cm2 or >0.4 cm2. While the group with an EROA >0.4 cm2 did not demonstrate a statistically significant benefit, the hazard ratio was 0.5 in favour of the MitraClip group, albeit with wide confidence intervals due to the small number of participants in that subgroup. All-cause mortality (24.3% versus 22.4%) and HF hospitalisation (48.7% versus 47.4%) were also similar in the device versus control arm, respectively. In the patients with available echo data during follow-up (n=89), there were no significant changes in end-diastolic volume, end-diastolic diameter, end-systolic volume, end-systolic diameter or LVEF. Recently published 2-year data showed similar results with no difference in all-cause mortality (33.9% versus 35%), cardiovascular death (31.2% versus 32.1%), unplanned HF hospitalisation (58.7% versus 63.5%) or the combined endpoint of all-cause mortality and HF hospitalisation (64.2% versus 68.6%). Additional follow-up to 2 years was available in all patients who were still alive and shows the consistency of previously published 1-year findings. Of note, the rate of first HF hospitalisation was lower in the MitraClip arm between 1 and 2 years although this was not statistically significant.14 In summary, the authors conducted an RCT of MitraClip versus medical therapy in a group of patients with severe HF, large and dilated ventricles and less than severe MR (defined according to current guidelines). MitraClip did not affect survival or HF hospitalisations at 1 or 2 years in patients who met inclusion criteria of MITRA-FR trial.
COAPT
The COAPT trial was a multicentre RCT in the US that randomised patients with symptomatic moderate–severe or severe MR to MitraClip and GDMT versus GDMT only.15 The trial randomised 614 patients over a period of 8 years and included 302 patients in the MitraClip arm and 312 patients in the GDMT arm. This final number of randomised patients (n=614) was increased from the initial target of 420 patients due to a higher than expected mortality in both arms. Key inclusion criteria of this trial were: moderate–severe (3+) or severe (4+) MR confirmed by an echocardiography core laboratory, EF 20–50%, LV end-systolic dimension ≤70 mm, at least one HF hospitalisation in the last year and/or elevated B-type natriuretic peptide (BNP) >300 pg/ml adjusted for BMI, and not a candidate for mitral valve surgery at the enrolling centre. Patients with ACC/AHA stage D HF, haemodynamic instability requiring inotropic or mechanical circulatory support, evidence of right-sided congestive HF with moderate/severe right ventricular dysfunction and pulmonary artery systolic pressure >70 mmHg were excluded.
The mean age of participants was 72 years, 64% were men and 61% had ischaemic cardiomyopathy. Mean values for echocardiographic data were 101 ml/m2 for LVEDV index, 31% for LVEF, 6.2 cm for LVESD and 0.41 cm2 for EROA. The surgical risk was not high in one-third of the patients. The Society of Thoracic Surgeons (STS) score for the risk of death within 30 days after mitral-valve replacement was 7.8% in MitraClip arm versus 8.5% in GDMT arm. NYHA class II HF was present in 39% of the patients and NYHA class III was present in 52% of the patients. The percentage of patients on diuretics, ACEI, ARB or ARNI, and beta-blockers was similar to the MITRA-FR trial, however exact dosages were not available for comparison. MitraClip was not attempted in six patients. Of the 293 undergoing MitraClip, technical success was achieved in 98% of the patients.
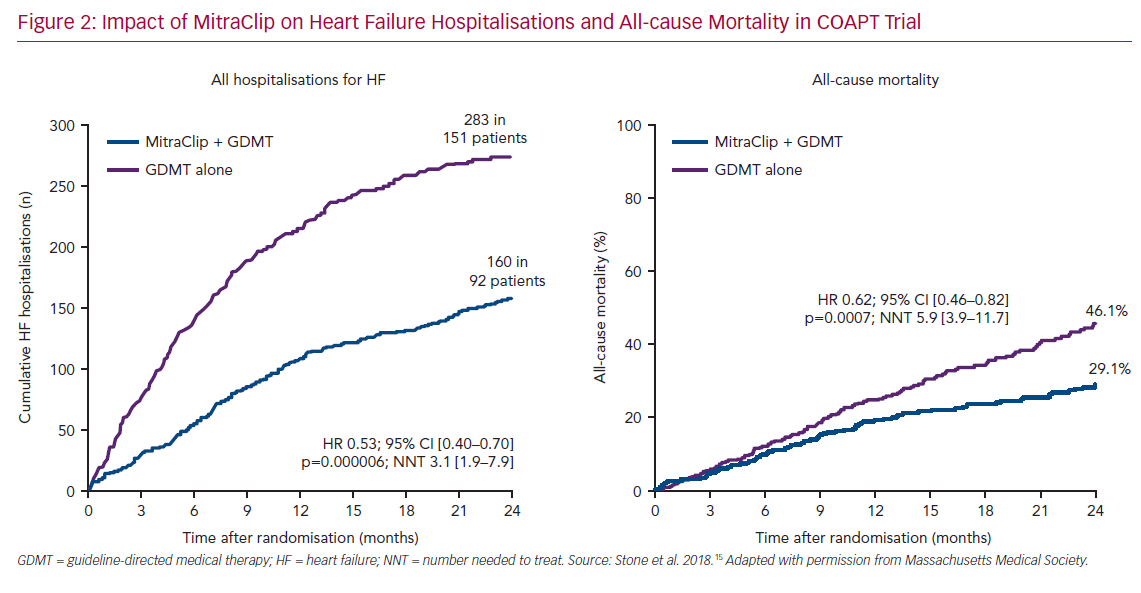
At discharge, 82% had <1+ MR and 12.7% has ≤2+ MR. The MR grade was ≤2+ in 92.7% patients at 30 days, 93.8% at 6 months, 94.8% at 1 year and 99.1% at 2 years. The primary endpoint of all HF hospitalisations at 2 years was significantly reduced with MitraClip (35.8%) compared with GDMT alone (67.9%; HR 0.53; 95% CI [0.40–0.70]), translating into a number needed to treat (NNT) of 3.1. (Figure 2). All-cause mortality was also significantly reduced at 2 years with MitraClip versus GDMT (29.1% versus 46.1%; HR 0.63; CI 95% [0.46–0.82] – NNT 5.9). The results were similar in all the pre-specified subgroups based on age, sex, LVEF, LVEDV and surgical risk. The incidence of LVAD or heart transplant was significantly lower in the device arm compared with GDMT alone (4.4% versus 9.5%). All other secondary endpoints such as MR grade reduction, quality of life and NYHA class were significantly lower in the MitraClip group. The mean LVEDV increased by 17.1 ml in the control arm compared with a reduction of 5.1 ml in patients receiving the MitraClip (p<0.001). In summary, this large randomised trial reported that MitraClip reduced the rate of HF hospitalisations and all-cause mortality and improved 6-minute walk distance and quality of life in patients with moderate-severe or severe SMR who were carefully screened to determine that they were taking maximally tolerated GDMT.
A recent sub-analysis of the COAPT data showed that reduction of MR to ≤2+ by either MitraClip plus GDMT or GDMT alone was associated with a significant reduction in risk of HF hospitalisation or all-cause mortality compared with MR ≥3+ (73.5%). There was no significant difference in this combined outcome between 0/1 (38.6%) and 2+ (49.8%) MR groups. At 30 days, 92.7% of the patients in the MitraClip arm had ≤2+ MR compared with 34.3% patients in the GDMT alone arm. In the MitraClip arm, the reduction in MR grade was stable at 2 years follow-up but in the GDMT alone group with ≤2+ MR at 30 days, an increase in degree of MR to ≥2+ was noted in 30% at 1 year and 66.7% at 2 years, emphasising the importance of close follow-up, early recognition and early treatment with MitraClip in patients who initially get better with GDMT alone.6
Consolidation of Data
MITRA-FR and COAPT trials randomised patients with SMR and HF to transcatheter mitral valve repair with MitraClip or GDMT alone. However, the results of these two trials are completely different. This has created vigorous discussion and speculation about the role of MitraClip in SMR. Explanations for the differences in the results of these trials include the following considerations.
Baseline Characteristics
Size of the Left Ventricle
The left ventricle was significantly more dilated at baseline in the MITRA-FR trial (average LVEDV index 135 ml/m2) compared with the COAPT trial (average LVEDV index 101 ml/m2). These trials do not provide a specific cut-off for clinical use. The impact of percutaneous mitral valve repair with MitraClip in patients with extremely large left ventricles and severe MR is unclear and needs further assessment. Based on the available data, using the COAPT criteria of LVESD <70 mm and EF >20% can be used as a starting point until more data is available.
Grade of Mitral Regurgitation Prior to MitraClip Placement
The mean EROA was 0.31 cm2 in the MITRA-FR trial compared with 0.41 cm2 in the COAPT trial. It can be postulated from the results of these trials that patients with a lower degree of MR may not benefit from this therapy as the change from pre-procedure to post-procedure will be less. In the COAPT trial, 255 patients were ineligible after initial screening because echocardiography criteria were not met which highlights the rigorous criteria for the trial.
The Hypothesis of More Benefit in ‘Disproportionate Versus Proportionate’ SMR
A widely discussed hypothesis that combines LV dysfunction as estimated by the LVEDV and the severity of the SMR using the EROA was proposed shortly after the publication of the two trials. The idea is that at any given LVEF and RV, SMR that is more severe than explained by the LV dysfunction is ‘disproportionate’ and is likely to respond to MitraClip therapy while proportionate SMR is not.16
Optimisation of Medical Therapy
The percentage of patients on GDMT was high and very similar in both the trials, however the exact dosages were not available. In the COAPT trial, two HF specialists shared the role of primary investigator and an HF expert was involved at each enrolling site for optimisation of medical therapy.15 On the other hand, local team members were asked to optimise HF as per ‘real world’ practice in the MITRA-FR trial.13 As optimised GDMT has a significant impact on mortality and hospitalisations, optimisation of GDMT before the procedure may have affected the results.
Procedural Characteristics
Procedural Complications
Procedural complications, including device implant failure, transfusion or vascular complication requiring surgery, atrial septal defect, cardiogenic shock, thromboembolism/stroke, tamponade or urgent surgery were reported in 14.6% of the patients in MITRA-FR. Device-related complications including thromboembolism, tamponade, vascular surgery, or urgent conversion to surgery occurred in 6.5% of patients. In COAPT, device-related complications were defined as any occurrence of single-leaflet device attachment, embolisation of the device, endocarditis that led to surgery, mitral stenosis (as confirmed by the echocardiographic core laboratory) that led to mitral valve surgery, implantation of a LV assist device, heart transplantation, or any other device-related event that led to non-elective cardiovascular surgery occurred in 3.4%.
Effectiveness of Mitral Regurgitation Reduction
Post-procedure ≥3+ MR was present in 9% of the MITRA-FR patients versus 5% of the COAPT patients. At 1 year, this was 17% versus 5%, respectively. Residual MR ≥3+ is independently associated with increased mortality and HF hospitalisations, and the consistent results in COAPT may explain better outcomes.
Post-procedural Characteristics
Consistency of Mitral Regurgitation Reduction
As stated above, reduced prevalence of 3+ MR in the MitraClip
arm during follow-up in the COAPT trial may explain better
long-term outcomes.
Close Follow-up with Heart Failure
Close follow-up with the HF team may have helped with further up-titration of GDMT in the COAPT trial. Further substudies from the COAPT trial will help answer this question.
Management of Secondary Mitral Regurgitation in the Contemporary Era
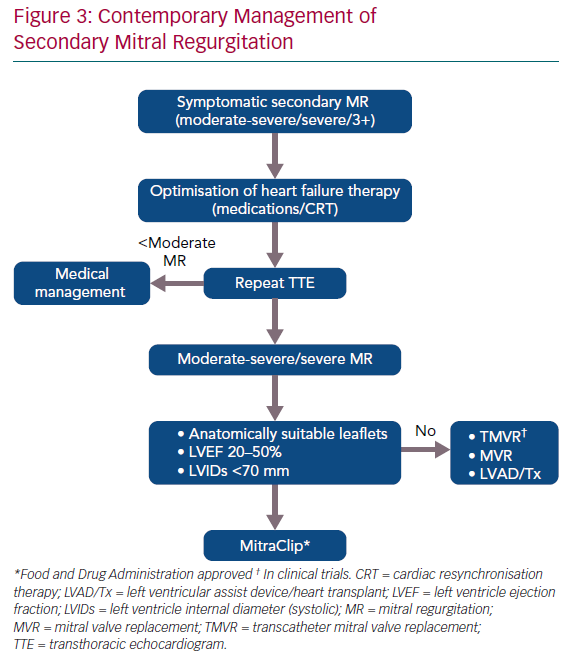
SMR is a growing problem and results in increased morbidity and mortality. Early recognition and treatment are the key elements in its management. The following steps may help optimise the management of these patients (Figure 3):
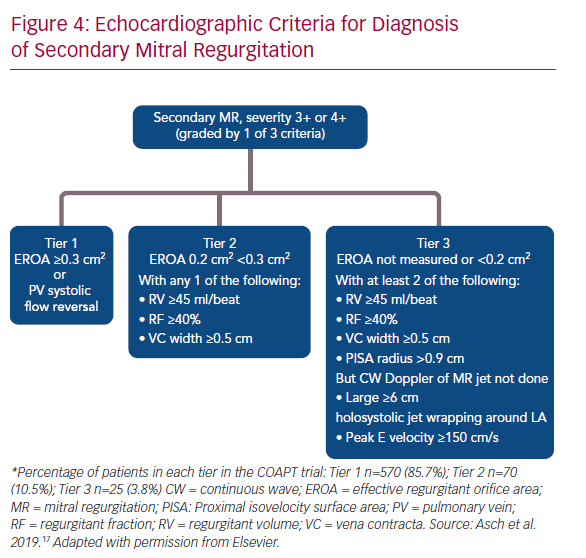
- Meticulous assessment of the mitral valve in patients with HFrEF with both quantitative and qualitative assessment of MR. All echocardiograms should report the LVEDV, EROA, RVol and RF, which will help the general cardiologist recognise the need for treatment. The echocardiographic algorithm reported by Asch et al. at the 2019 ACC meeting is likely to become the standard for assessment (Figure 4).17
- Involvement of HF team or specialists early in the care of these patients. Optimisation of medical therapy including the need for CRT is important, ideally on an outpatient basis with close follow-up.
- Right and left heart catheterisation to assess the filling pressures after medical optimisation. However, filling pressures should not determine the need for treatment as these are recorded at rest and may be higher with exercise.
- Consultation with an interventional/structural cardiologist to determine the candidacy for MitraClip.
- Optimisation with IV diuretics before and after the MitraClip.
- Continued follow-up with an HF specialist for management of HF.
Although there were only modest increases in GDMT in the MitraClip group compared with GDMT alone in COAPT due to the protocol instructions, it is quite likely that many patients who undergo transcatheter mitral valve repair with MitraClip will be able to tolerate higher doses of GDMT that were not previously tolerated. This would parallel the situation following CRT where the ability to up-titrate GDMT has been associated with significantly improved outcomes.18
Conclusion
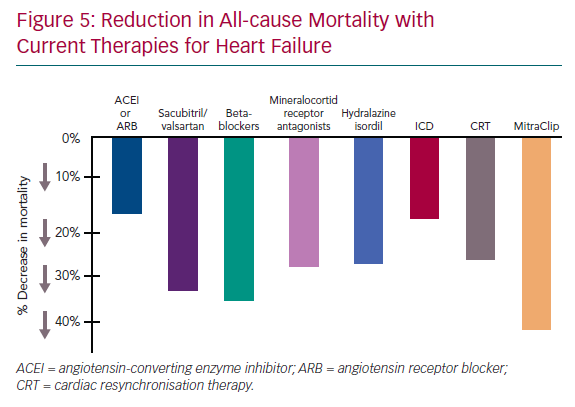
Transcatheter mitral valve repair with MitraClip is the new gold standard for treatment of SMR. Based on the COAPT trial data, the relative reduction in all-cause mortality with MitraClip is the highest of all the available therapies including medications or CRT as shown in Figure 5. It has proven that correction of SMR in HFrEF with MitraClip reduces mortality, reduces HF hospitalisations and improves quality of life, NHYA class and 6-minute walk distance. Patient selection is the key to identify those who will benefit the most from this therapy. Patients with large dilated left ventricles, ≤3+ MR, severe irreversible pulmonary hypertension, severe right ventricular dysfunction, or those on inotropic support may not benefit. It is important to remember that 29% of the patients in the MitraClip arm in COAPT had died by the 2-year follow-up, emphasising the high mortality associated with SMR. Therefore, we need to identify the factors that can help in early diagnosis and treatment and continue to enhance GDMT as allowed following the procedure.