Takotsubo syndrome is an acute reversible heart failure syndrome, which is increasingly recognised by coronary angiography for patients with acute ‘cardiac’ chest pain.1 It is a distinct disease entity from acute coronary syndrome, although the initial presentation has similar features to either ST elevation myocardial infarction (STEMI) or non-ST elevation myocardial infarction (NSTEMI). Early access to diagnostic coronary angiography has helped identify the increasing incidence of this condition and over the past 24 years there has been increase in the number of case reports, series and registries reported.2
Nomenclature
Various names have been used to describe the appearance now described as Takotsubo cardiomyopathy or Takotsubo syndrome, following the initial label given by Sato and colleagues in 1990, comparing the appearance of the left ventricle at end-systole to the local Japanese fishermen’s octopus pots in the Hiroshima fishing markets.3 Many names have been used for the condition, including stress or stress-induced cardiomyopathy, apical ballooning syndrome,4 ampullary-shaped cardiomyopathy5,6 and ‘broken-heart’ syndrome in the context of bereavement.7 While it is a form of acute heart failure, one of the characteristics contributing to the definition is the recovery of the dysfunctional myocardial segments. The majority of patients recover normal cardiac function and patients have a low risk of major adverse cardiac events. The term cardiomyopathy implies a primary disease of the cardiac muscle, but the full recovery and low major adverse cardiac event rate at follow up show major differences with the primary cardiomyopathies. The consensus is that as the diagnosis of Takotsubo syndrome is currently made based upon clinical observations, it fulfils the definition of a clinical syndrome rather than a cardiomyopathy.
Definition
Takotsubo syndrome and its associated variants constitute a type of acute, reversible heart failure that may represent a form of acute catecholaminergic myocardial stunning in the absence of culprit occlusive coronary artery disease to explain the pattern of temporary left ventricular (LV) dysfunction.8–11 Several diagnostic criteria strategies have been proposed, including those by the Mayo Clinic (modified in 2008), the Japanese Takotsubo Cardiomyopathy Group, the Gothenburg Group and the Takotsubo Italian Network.12–16 The author was part of the working group that developed the new 2014 European Society of Cardiology (ESC) Takotsubo Syndrome Diagnostic Criteria (see below). It is important to note that whilst this condition predominantly affects post-menopausal women (~90 % of all cases reported, particularly in the larger cohorts), men and younger women can also from suffer the condition and therefore demographic features are not a mandatory part of the proposed diagnostic criteria.
Diagnostic criteria
- Transient regional wall motion abnormalities of the left and/or right ventricular (RV) myocardium, which are frequently, but not always, preceded by a stressful trigger (emotional or physical).
- Regional wall motion abnormalities usually extending beyond a single epicardial vascular distribution, often resulting in circumferential dysfunction of the ventricular segments involved (apical and/or mid- LV or basal segments).
- Absence of culprit atherosclerotic coronary artery disease including acute plaque rupture, thrombus formation and coronary dissection or other pathological conditions to explain the pattern of temporary LV dysfunction observed (e.g. hypertrophic cardiomyopathy, viral myocarditis).
- New and reversible electrocardiogaphy (ECG) abnormalities (ST-segment elevation, ST depression, left bundle branch block, T-wave inversion and/or QTc prolongation) during the acute phase.
- Significantly elevated serum natriuretic peptide (B-type natriuretic peptide [BNP] or N-terminal pro b-type natriuretic peptide [NTproBNP]) level during the acute phase.
- Positive but relatively small elevation in cardiac troponin measured using a conventional assay (i.e. disparity between the troponin level and the amount of the dysfunctional myocardium present).
- Recovery of ventricular systolic function on cardiac imaging at follow up.
Clinical Subtypes – Primary and Secondary Takotsubo Syndrome
The medical community has reported a variety of clinical scenarios and contexts in which patients with Takotsubo syndrome present to medical attention. These can be classified into two groups:
Primary Takotsubo Syndrome
Primary Takotsubo syndrome occurs in individuals when the specific symptoms described are the primary reason for their acute presentation. These include patients with or without clearly identifiable stress triggers (these are often emotional) and any potential co-existing medical conditions that may serve as predisposing risk factors, but are not the primary cause for the catecholamine rise. These cases can be considered primary Takotsubo syndrome, with clinical management directed to the specific complications.
Secondary Takotsubo Syndrome
A significant proportion of cases occur in individuals already hospitalised for other medical, surgical, anaesthetic, obstetric or psychiatric conditions. These individuals have a sudden activation of their sympathetic nervous system and/or a rise in catecholamines and develop an acute Takotsubo syndrome as a complication of their primary condition or its treatment. These should be diagnosed as secondary Takotsubo syndrome, thereby focusing on the management pathway not only for Takotsubo syndrome and its cardiac complications, but also for the primary underlying disease and its treatment that served as the trigger for the secondary Takotsubo syndrome.
Anatomical Variants
Primary and secondary Takotsubo syndromes can present with an array of possible anatomical variants.17–19 The initial definition of Takotsubo syndrome described what is now considered the classic pattern of LV regional wall motion abnormalities, with apical and circumferential mid-ventricular hypokinesia and basal hypercontractility. At end-systole the left ventricle has the typical appearance of the Takotsubo with a narrow neck and globular lower portion, giving the appearance of virtual ‘apical ballooning’. This typical Takotsubo syndrome variant with apical dysfunction is present in ~50–80 % cases depending on the various series reported, but a number of other anatomical variants may also occur. The two most common atypical variants are the inverted Takotsubo or basal variant, with circumferential basal hypokinesia and apical hypercontractility – also referred to as the ‘nutmeg’ or ‘artichoke’ heart – and the mid-ventricular variant with circumferential mid- ventricular hypokinesia and both basal and apical hypercontractility.20–22 This has a unique end-systolic appearance which has been likened to either a Greek vase or the ‘ace of spades’, although the basal variant also can have the ace of spades appearance. In both inverted and mid-LV Takotsubo variants, the similar principle exists of reversible LV dysfunction affecting more than one coronary territory, usually circumferential pattern, in the absence of culprit coronary artery disease.
Other rarer variants have been described, including biventricular apical dysfunction, dysfunction sparing the apical tip (possibly a form of the mid-ventricular Takotsubo variant) and isolated RV Takotsubo syndrome.19,23–25 These different morphological variants may depend upon the timing of early segment recovery and clinical evaluation. Recurrent cases have been described with different anatomical variants in the same individual, suggesting that an individual can be susceptible to more than one subtype.26,27
Epidemiology
Several series in Asian and Western, predominantly Caucasian, populations suggest around 1–2 % of patients with suspected acute coronary syndrome (ACS) are eventually diagnosed with Takotsubo syndrome.17,28 With increasing awareness and more widespread access to early coronary angiography, the syndrome is now being recognised and appreciated more frequently.
In the first study, Takotsubo syndrome was diagnosed in 0.02 % of all acute hospitalisations (6,837/33,506,402 patients).29 The majority were elderly post-menopausal women (90 %, aged from 66–80 years), a demographic repeated across many published cohorts, with risk factors including smoking, alcohol abuse, anxiety states and hyperlipidaemia. A higher Takotsubo syndrome rate was observed in whites compared with African Americans and Hispanics (67.4 % versus 4.4 % and 4.3 %, respectively).29 The second study and largest cohort to date reported details of 24,701 patients with a discharge code for Takotsubo syndrome. The study found similar demographics with 89 % women, with a mean age of 66.9 ± 30.7 years, and most patients (59.6 %) were ≥65 years old.30
Gender Differences
Takotsubo syndrome predominantly affects post-menopausal women. In the German Takotsubo syndrome registry (324 patients; 91 % female and 9 % male with a mean age of 68 ± 12 versus 66 ± 12 years, respectively), both genders showed similar demographic and clinical characteristics.8 Emotional stress or no identifiable trigger were more prevalent in triggering episodes in women. Conversely a physical stress-triggering event, shock and/or resuscitation on presentation and higher levels of cardiac biomarkers (troponin), QT prolongation were more frequent in men.
The National Inpatient Sample USA cohort (2008–2009; 24,701 Takotsubo syndrome patients) reports significantly higher mortality rates in male (8.4 % male versus 3.6 % females; p<0.0001), perhaps reflecting the higher frequency of underlying severe critical illness and secondary Takotsubo syndrome (36.6 % in men versus 26.8 % in women; p< 0.0001).30
Age
Elderly patients are at higher risk of Takotsubo syndrome and related major complications, whereas fewer than 10 % of patients are below 50 years of age.29,31 In the Takotsubo Italian Network, Takotsubo syndrome patients older than 65 years have greater prevalence of hypertension, cerebrovascular disease, a lower glomerular filtration rate and LVEF at discharge. Older adults (≥75 years) have higher in-hospital complications and in-hospital mortality rates (6.3 % versus 2.8 % of overall in-hospital mortality).9
Pathophysiology
Given the frequently sudden, unexpected stressful precipitant, the signs of sympathetic activation at presentation and the secondary medical triggers that also lead to extreme sympathetic activation, the role of catecholamines appears central to the pathophysiology of Takotsubo cardiomyopathy. Serum catecholamine levels at presentation are significantly elevated compared with both resting levels in the individual patient and to levels in comparable patients with acute heart failure secondary to acute myocardial infarction.32 Several varieties of iatrogenic Takotsubo syndrome cases have been reported after administration of sympathomimetic drugs; for example, dobutamine in stress echocardiography.33
Several hypotheses have been proposed to explain the unique cardiac appearance in Takotsubo syndrome and the cardiac response to severe stress. These can be broadly divided into vascular and myocardial causes.
Acute Multivessel Coronary Spasm
The initial cases described in Japan frequently had concomitant vasospasm at diagnostic coronary angiography and it is conceivable that some individuals may be prone to multivessel coronary artery spasm. Some authors propose that Takotsubo syndrome results from multivessel vasospasm and is a form of ischaemic stunning with superimposed catecholamines.34 In some cases vasospasm correlates with the region of dysfunction, but equally in other cases it does not, which goes against vasospasm as the cause of Takotsubo syndrome.35 There are also significant differences in histopathological features when examining endomyocardial biopsies taken from patients with Takotsubo syndrome that show a pattern of myocardial abnormalities not associated with infarcted, stunned or hibernating myocardium, which would not support a primary vascular cause.36 All patients showed the typical contractile pattern of Takotsubo and complete functional recovery within 12 ± 3 days. In ‘acute’ biopsies, many vacuoles of different sizes were found with intracellular accumulation of glycogen. Structural deteriorations characterised by disorganisation of contractile and cytoskeletal proteins could be seen, but evidence of oncotic and apoptotic cell death, as well as infarction, were absent.
Acute Left Ventricular Outflow Tract Obstruction
Acute LV outflow tract obstruction (LVOTO) has been proposed as the cause for Takotsubo syndrome. Individuals may develop a dynamic mid- cavity LV obstruction under catecholamine excess. Potentially older women with smaller hearts, who frequently have prominent septal bulges, could be predisposed to acute LVOTO during intense stress and sympathetic activation. This could explain the transient nature of the regional dysfunction as the apical wall stress would subsequently be reduced by the ensuing ischaemia and apical dysfunction, limiting pressure necrosis and infarction. LVOTO was noted in 25 % of cases in the acute phase of Takotsubo syndrome,37 which indicates that it can be a contributory factor in a subset of patients but is unlikely to be the underlying main cause of Takotsubo syndrome.
Direct Catecholamine-mediated Myocardial Stunning
A consistent finding across several mammalian species, including humans, is a higher sympathetic nerve density in the basal myocardium compared with the apex. Conversely, there is an apical-basal gradient of adrenergic receptors ( AR). These opposing sympathetic nerve andAR gradients allow balanced myocardial responses to sympathetic activation under low and medium levels of activation, but at the highest levels epinephrine release from the adrenal glands would perfuse the heart via the circulation and the receptor gradient would determine the response. The second element of the hypothesis proposed is that epinephrine at low and medium doses is a positive inotrope, but at the highest doses it becomes a negative inotrope via the 2AR, by activating a switch from the Guanine Nucleotide binding protein stimulation to the cardioinhibitory Guanine Nucleotide binding protein inhibition (Gi) secondary messenger.38,39 This could explain the transient negative inotropism observed in the apical myocardium after exposure to very high levels of circulating epinephrine, such as those reported in Takotsubo syndrome patients following triggers of extreme stress. Activation of the 2AR-Gi pathway is cardioprotective and may minimise the toxic effects of excessive catecholaminergic stimulation upon the myocardium. This hypothesis was tested in a rodent model in which high-dose epinephrine induced reversible apical hypokinesia and it was found this Takotsubo model could be prevented by prior treatment with pertussis toxin, which deactivates Gi signalling.40
Combinations of these hypotheses form the most likely causes of the cardiac response to severe stress.41
Clinical Presentation and Diagnosis
Individuals with Takotsubo syndrome typically present with acute and unstable chest pain of cardiac origin (angina), breathlessness, palpitations due to sinus tachycardia or arrhythmia and in the more severe cases presyncope or syncope secondary to ventricular tachyarrhythmias, severe LVOTO and/or cardiogenic shock. The majority of diagnoses of Takotsubo syndrome are made at cardiac catheterisation when diagnostic coronary angiography demonstrates the absence of culprit coronary artery disease to explain the presenting symptoms, ECG changes and regional wall motion abnormalities.
Once the diagnosis of Takotsubo syndrome is confirmed, early cardiac imaging and cardiac biomarkers are helpful to exclude myocardial infarction and to further stratify risk. This strategy is to be recommended to allow patients with a higher-risk phenotype to be kept under observation with appropriate monitoring in a coronary care or high- dependency care setting, while those with lower risk can be cared for in a non-specialist ward and discharged once baseline imaging and risk stratification has been completed and the patient is asymptomatic, free of arrhythmias and haemodynamically stable.
When the clinical picture is uncertain, cardiac magnetic resonance imaging with late gadolinium enhancement often clarifies the presence or absence of acute myocardial infarction in a typical coronary distribution.18,19
Pathology
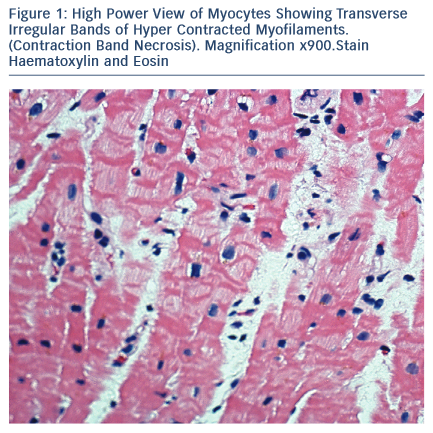
The majority of Takotsubo syndrome cases survive. The rare cases that have undergone biopsy during the acute phase show contraction- band necrosis in myocytes as evidence of catecholamine-triggered cardiomyocyte calcium overload (see Figure 1).42 This can be can be fatal due to the complications of cardiogenic shock, arrhythmia or cardiac rupture and can be readily detected post-mortem in a confirmed case of Takotsubo syndrome. More difficult is the case of sudden cardiac death in the community with a stressful precipitant and a structurally normal heart and coronary arteries.43–45
In addition to inherited arrhythmic sudden death syndromes known to be triggered by stress (e.g. catecholaminergic polymorphic ventricular tachycardia), acute Takotsubo syndrome with ventricular fibrillation or asystole should be considered if the heart is normal. There may be dilatation or oedema in the distribution characteristic of an anatomical variant of Takotsubo syndrome, but if post-mortem examination is delayed then non-specific contraction may prevent this assessment.46–48 A hypercontracted left ventricle at autopsy, with increased wall thickening, but a normal heart weight has been reported in the context of severe stress (death in systole). Contraction-band necrosis of individual myocytes with interstitial oedema and a mixed inflammatory cell infiltrate are described in this phenomenon.42,49,50 Cases of idiopathic infarction with normal coronary arteries resulting in sudden death may also be due to Takotsubo.51
Complications and Acute Prognosis
A variety of complications can occur in ≤52 % of Takotsubo patients.8,52,53
Right Ventricular Involvement
Patients with biventricular involvement generally take a more severe clinical course. RV involvement assessed by ECG or magnetic resonance imaging has been reported in 18–34 % of the patients and is associated with older age, a lower LV ejection fraction, a higher frequency of heart failure, pleural effusion and a longer hospital stay.8,54
Acute Heart Failure
Systolic heart failure is the most common complication in the acute phase of Takotsubo syndrome, occurring in 12–45 % of patients.8,16,28,53,55 Independent predictors for the development of acute heart failure are advanced age, low LV ejection fraction on presentation, higher admission and peak troponin levels and a physical stressor preceding the onset of Takotsubo syndrome. In some patients pulmonary oedema due to acute LV dysfunction is aggravated by mitral regurgitation and/or LV outflow tract obstruction.
Left Ventricular Outflow Tract Obstruction
As a consequence of myocardial stunning of the apical segments and hypercontraction of the basal LV myocardium, a dynamic intraventricular pressure gradient due to mitral valve systolic anterior motion may develop in the acute phase. Significant LV outflow tract obstruction with gradients ranging from 20–140 mmHg have been observed in 10–25 % of patients, often accompanied by mitral regurgitation.28,53,56 A mid- ventricular or LVOTO gradient >25 mmHg is considered haemodynamically significant and ≥40 mmHg is a high-risk factor. Abnormal Q waves in the electrocardiogram, hypotension and cardiogenic shock are more frequent in these patients. Normally, the outflow tract obstruction resolves spontaneously over a few days.
Mitral Regurgitation
Acute mitral regurgitation is another potentially serious complication occurring in 14–25 % of patients.57,58 LV ejection fraction is lower and pulmonary artery pressure higher in cases with significant regurgitation and these patients present more frequently with acute heart failure or cardiogenic shock. Two independent mechanisms may cause acute mitral regurgitation: systolic anterior motion of the mitral valve in association with dynamic LV outflow tract obstruction and apical tethering of the subvalvular mitral valve apparatus.59 In most cases the mitral regurgitation improves with normalisation of LV function, which may be delayed compared with patients without acute mitral regurgitation.
Cardiogenic Shock
Cardiogenic shock, primarily due to acute LV dysfunction, may be aggravated by RV involvement, LV outflow tract obstruction or acute mitral regurgitation. Echocardiography plays an important role in determining the mechanism of cardiogenic shock in order to apply an appropriate therapy. The mortality of cardiogenic shock in Takotsubo syndrome is high (between 17–30 %).8,41,53,55,60
Arrhythmias
New onset of atrial fibrillation has been reported in 5–15 % of patients with Takotsubo syndrome.8,55,61,62 During the acute phase of Takotsubo syndrome, ventricular arrhythmias occur in 4–9 % of patients. Resuscitation as a result of cardiac arrest, which may be the initial presenting symptom, has been reported in 4–6 % of cases. Bradycardia due to atrioventricular block and asystole has been described in a limited number of patients (2–5 %).
Thrombus formation
Thrombus formation may be detected within the akinetic ventricular apex in 2–8 % of Takotsubo syndrome patients, occasionally resulting in stroke or arterial embolism.63
Pericardial Effusion
Acute pericarditis with recurrent chest pain, reappearance of ST-segment elevation and a small amount of pericardial effusion has been observed in some patients during the recovery phase of Takotsubo syndrome.64–66
Ventricular Wall rupture
Mechanical complications including rupture of the ventricular free wall or perforation of the interventricular septum are seen in less than 1 %.67,68
Mortality
Initial small case series have reported mortality rates varying from <1–12 %.17,69 In large studies and registries, in-hospital mortality is lower and has been observed in 2–5 % of the patients with Takotsubo syndrome, mainly caused by refractory cardiogenic shock or ventricular fibrillation.8,53 A recent meta-analysis evaluating 37 studies with 2,120 patients reported an in-hospital mortality of 4.5 %70 and this figure matches the 4.2 % in-hospital mortality reported in the large NIS- USA Takotsubo syndrome cohorts in 2008 to 2009.29,30
Recurrence
The reported incidence of recurrence varies between 3.5–22 % of cases from different series.13,16,41,55 If a patient has a recurrent episode of Takotsubo syndrome, they are once more at risk of complications. Prognosis should be individualised considering the likelihood of recurrence, triggering event and co-existing medical conditions. If an individual has a recurrent episode then long-term clinical follow up should be considered.
Long-term Prognosis
Data regarding long-term prognosis of patients with Takotsubo syndrome are limited, but initial reports from limited cohorts suggest prognosis may be similar to acute myocardial infarction, but driven by non-cardiovascular causes and higher than the healthy age- matched population.
Most patients settle rapidly following the acute episode and become asymptomatic. There is increasing evidence of physiological abnormalities persisting beyond the timeframe when resting contractile abnormalities have normalised macroscopically. There is also growing recognition of a subgroup of patients with persistent cardiac symptoms following the acute episode.71 These include angina, exertional breathlessness, palpitation and/or a tremulous anxiety state, reflecting heightened sympathetic tone. Although the coronary arteries are unobstructed and ventricular function has recovered macroscopically, it is helpful to document objective evidence of ongoing cardiac abnormalities to reassure the patient and to guide treatment. Twenty-four-hour Holter ECG monitors to assess for atrial arrhythmias or inappropriate sinus tachycardia (continuous or paroxysmal) and 24-hour ambulatory blood pressure monitors may be helpful for detecting transient and inappropriate hypertensive episodes. Persisting ECG changes and sometimes other evidence of autonomic disturbance can provide objective evidence and exclude non-cardiac explanations for ongoing symptoms.
Clinical Management
General Considerations
One of the most important criteria for the diagnosis of Takotsubo syndrome is eventual spontaneous restoration of normal cardiac function. The major objective of in-hospital treatment should be supportive care to sustain life and to minimise complications during recovery. In mild cases, either no treatment or a short course of limited medical therapy may be sufficient. In severe cases complicated by progressive circulatory failure and cardiogenic shock, the patient should be considered for early mechanical support as a ‘bridge-to-recovery’.
Conclusion and Future Directions
Takotsubo syndrome is a fascinating acute heart failure syndrome increasingly recognised by the medical community. Many facets of this condition are incompletely understood and current knowledge to guide optimal clinical management is limited. The increasing incidence and the high frequency of complications during the acute phase underpin the need to improve care pathways for individuals with Takotsubo syndrome with establishment of national and international registries.