Statins are currently the most efficacious and widely prescribed lipid-lowering medications.1 Numerous randomised controlled trials across a spectrum of baseline atherosclerotic cardiovascular disease (ASCVD) risk show that a 1.0 mmol/L (~40 mg/dL) reduction in low-density lipoprotein cholesterol (LDL-C) with statin therapy is associated with an overall 21 % reduction in major vascular events and 20 % reduction in coronary death.2 Prescribing behaviour of statins has dramatically changed in the last 20 years, with a sixfold increase of statin use in 18–64 year olds and an eightfold increase in adults >65 years old in the US.1 Globally, in the Organisation for Economic Co-operation and Development (OECD) countries, the use of cholesterol-lowering medications including statins has tripled since 2000.3 The recent release of the 2013 ACC/AHA cholesterol guidelines provided a dramatic shift in the treatment approach of cholesterol from the previous Adult Treatment Panel III (ATP III) guidelines, with a focus on fixed dose moderate/high intensity statin treatment.4 According to the new guidelines, the number of eligible individuals for statin treatment is estimated to increase. In comparison, the 2011 ESC/EAS guidelines for cholesterol management risk stratify patients into four risk groups: very high, high, moderate or low risk based on the Systematic Coronary Risk Evaluation (SCORE) risk chart and target specific LDL-C goals.5 The various guidelines and increasing use of statins presents a challenge for providers and a need for practical considerations for use.5,6 It still remains unclear how the ACC/AHA guidelines will affect practice in Europe.
Considerations for Specific Disease States
Primary Prevention
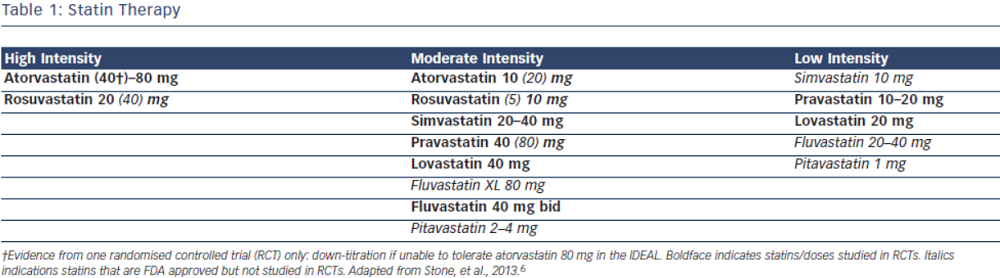
Current evidence supports the use of statins in individuals without vascular disease at higher ASCVD risk for the primary prevention of future cardiovascular events.7–9 In the most recent meta-analysis which included ~60,000 individuals by Taylor et al., authors concluded statin therapy is associated with a 14 % reduction in all-cause mortality and a 35 % reduction in combined fatal and non-fatal cardiovascular disease, coronary heart disease and stroke.8 The 2013 ACC/AHA cholesterol guidelines recommend use of high-intensity or moderate-intensity statins (see Table 1) in individuals with 10 year ASCVD risk of 7.5 % or greater using the pooled cohort equation (PCE). In individuals with a PCE score of 5–7.5 % consideration should be given to treatment with moderate dose statin based on the presence of other risk factors such as family history, LDL-C level, inflammatory markers, coronary calcium score, ankle-brachial index and lifetime risk.6 Decision to choose between high-intensity and moderate-intensity statin can be taken by the individual provider, weighing the risk/benefit ratio of statin side effects. Elderly patients and those with a history of myalgia or risk factors for statin intolerance should be started on moderate-intensity statins.10–13 However, patients with LDL>190 should be on high intensity statins.6 ESC/EAS guidelines recommend treatment with statin for patients in the high-risk group and targeting goal LDL-C levels <70 mg/dL (2.5 mmol/L) for high-risk groups based on SCORE chart or those with LDL-C levels >190 mg/dL (4.9 mmol/L).5
Atherosclerotic Cardiovascular Disease
The 2013 ACC/AHA guidelines recommend use of high-intensity statins in individuals with known ASCVD, specifically atorvastatin 80 mg or rosuvastatin 20–40 mg.6 In the Cholesterol Treatment Trialist (CTT) Collaboration meta-analysis including over 170,000 individuals, the authors concluded that more intensive statin therapy and lower LDL-C levels resulted in further reductions in major cardiovascular events. High-intensity statin therapy demonstrated 15 % reduction in major cardiovascular events as compared to low intensity statins. Overall, authors found that all-cause mortality was reduced by 10 % per 1.0 mmol/L reduction in LDL-C.2 In patients with ASCVD the evidence overwhelmingly supports treatment with high dose statins such as atorvastatin 80 mg or rosuvastatin 20–40 mg daily regardless of baseline LDL-C levels. The ESC/EAS guidelines approach ASCVD as high risk and recommend targeting LDL-C levels <70 mg/dL (<1.8 mmol/L) or at least a 50 % reduction in LDL-C. These guidelines also recommend combination therapy if target cannot be reached with statin only.5
Chronic Kidney Disease
Currently under the new ACC/AHA 2013 cholesterol guidelines, chronic kidney disease (CKD) is not classified as a coronary heart disease (CHD) equivalent and does not factor into the risk calculator.6 This differs from ESC/EAS guidelines which place CKD patients into the high risk category.5 Studies have shown increased cardiovascular events and mortality in with patients with decreased estimated glomerular filtration rate (eGFR) and albuminuria.14,15 Independent of lipid effects, a meta-analysis, which included 6,452 CKD patients randomised to statin versus placebo, showed renoprotective effects of statins. Statin therapy was associated with significant reductions in urinary protein excretion (0.77 g/24 h) and creatinine (0.65 mg/ dl), but improvement in creatinine levels was only associated with long-term statin use (>3 years). Significant increase in eGFR was also found between 1–3 years on statin therapy, but this improvement in renal function did not persist beyond three years.16 The Study of Heart and Renal Protection (SHARP) trial directly assessed the cardiovascular benefit of statins in patients with CKD. This study randomised 9,438 adults to the combination simvastatin 20 mg/ ezetimibe 10 mg versus placebo and found a 17 % reduction in cardiovascular events in those allocated to statin/ezetimibe.17 This benefit was not completely from statins, as previous studies have demonstrated the efficacy of ezetimibe added to statins. A recent meta-analysis comparing combination therapy versus statin monotherapy included 11 studies on ezetimibe and concluded an additional 18 % reduction of LDL-C with the addition of ezetimibe.18 These findings are reflected in the new guidelines from the Kidney Disease: Improving Global Outcomes (KDIGO) group, which recommends statin/ezetimibe combination therapy for adults >50 years old with eGFR<60 ml/min/1.73 m2 who are not treated with chronic dialysis or kidney transplantation. In adults aged 18–49 with CKD, consideration should be given to statins if they also have risk factors such as known CHD, diabetes mellitus, prior ischaemic stroke or elevated cardiovascular risk score.19 Atorvastatin is recommended in CKD because of its favourable pharmacokinetics and minimal renal excretion negating need for dose adjustment. Rosuvastatin can also be used safely in patients with advanced CKD (GFR<30 ml/ min/1.73 m2), but should be started at 5 mg daily and titrated up to maximum of 10 mg daily if needed.20
Patients on chronic dialysis have been directly studied in randomised controlled trials and appear not to benefit from statin therapy. In the 4D study, 1,255 patients with diabetes mellitus receiving maintenance haemodialysis were randomised to atorvastatin 20 mg or placebo with no difference in cardiovascular death, stroke or non-fatal myocardial infarction (MI) in the two groups after a median follow-up of four years.21 A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events (AURORA) randomised 2,776 patients on maintenance haemodialysis to rosuvastatin 20 mg versus placebo and again found no benefit in the statin group.22 Therefore, statins should not be initiated in patients on maintenance haemodialysis.
Diabetes Mellitus
Diabetes Mellitus (DM) is associated with increased risk of cardiovascular diseases23,24 and early onset is considered a CHD risk equivalent.25 Current guidelines recommend treatment with at least moderate intensity statin for all adults >40 years old and high intensity statin for those with a 10 year risk score >7.5 %.6 ESC/EAS guidelines recommend statins for patients with diabetes only when they develop evidence of end organ damage.5
Dosing, Low-density Lipoprotein levels and Statin Intolerance
LDL-C targets have been abandoned in the ACC/AHA guidelines and instead using fixed doses of high and moderate intensity statins are recommended. The intensity of the statin dose is matched to the person’s baseline ASCVD risk. Although these guidelines make no recommendations for or against targeting specific LDL levels, they do suggest follow up assessment of the lipid panel to assess compliance.6,26 In contrast, the ESC/EAS guidelines approach cholesterol management with targeted LDL-C levels for specific disease states and have no specific statin intensity recommendations.5
Statin-associated Myalgias
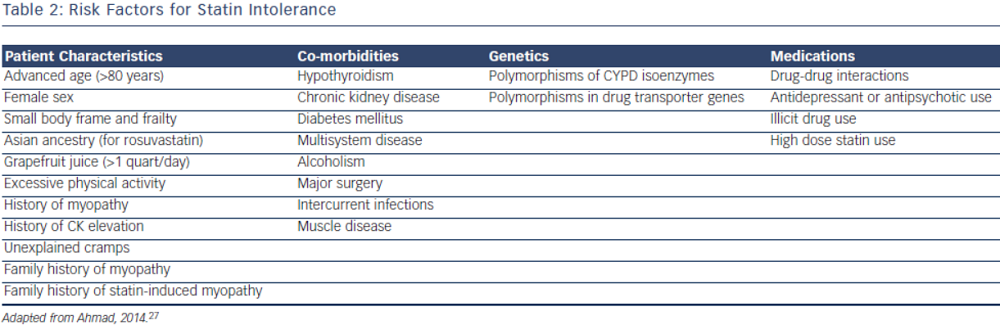
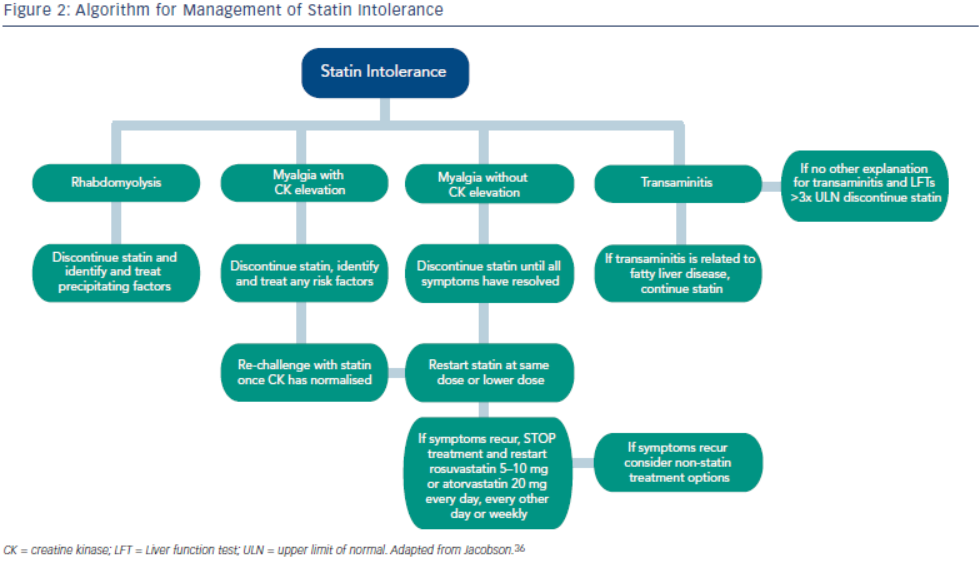
Statin intolerance or the inability to continue statins due to adverse symptoms or abnormal lab values occurs in roughly 5–10 % of patients who use statins. There are only a limited number of studies on statin intolerance creating a diagnostic and therapeutic challenge to prescribers.27 Observational studies like the Prediction of Muscular Risk in Observational conditions (PRIMO) survey, which included 7,924 patients on high dose statins in the outpatient setting found 10.5 % of patients developed muscular symptoms, with the average onset of symptoms being ~1 month after initiation of the statin.28 However, the incidence of reported statin intolerance is greatly variable and thus far randomised clinical trials of statins versus placebo have not shown major differences in symptoms.29,30 Most statin intolerance due to muscle symptoms is related to myalgias, defined as muscle aches without creatinine kinase CK elevation, but rarely patients have developed clinically significant myopathies. Several risk factors have been identified for statin intolerance (see Table 2) and though there is no clear consensus on management of statin intolerance, several small studies have explored this issue. For patients with frank rhabdomyolsis statin therapy should be discontinued.27 For patients with muscle symptoms but normal enzymes the ACC/AHA cholesterol guidelines recommend withdrawal and rechallenge with statin. The patients should be rechallenged with statin only after all muscular symptoms have resolved, which may take >2 weeks.6,27 With regard to rechallenging, Glueck et al.31 found that 60/61 patients enrolled tolerated rosuvastatin 5 or 10 mg daily. Similarly, another study by Stein et al.32 enrolled patients who had discontinued statins in the past due to muscle-related side effects or those who were currently on statin therapy but had impaired quality of life, they found that 97 % of patients tolerated fluvastatin. In addition to switching statin therapy, there has been investigation into alternate doing strategies. A retrospective analysis by Backes et al. found that 72.5 % of the patients tolerated every other day 5–10 mg rosuvastatin.33 Kennedy et al. found that 80 % patients tolerated once weekly 5 or 10 mg of rosuvastatin.34 Simvastatin 80 mg should be avoided in general due to increased risk of myopathy and lack of incremental efficacy compared to lower doses.35 Algorithms for managing patients with statin intolerance may facilitate decision-making in the clinic (see Figure 1).36
Statin-associated Transaminitis
Elevations of serum alanine aminotransferase or aspartate aminotransferase up to 3x the ULN transaminitis is also seen with statin users in 0.5–3 % of patients.37 In a post hoc analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study population, 437 patients with baseline elevation of liver enzymes, thought to be related to fatty liver disease, were randomised to placebo versus statin therapy. The authors found an improvement of liver enzymes and 68 % risk reduction of cardiovascular events in the group treated with statin therapy.38 Similarly in a post hoc analysis of the Incremental Decrease in Events through Aggressive Lipid Lowering (IDEAL) study, authors found that 1,081 patients with ALT>Upper Limit of Normal (ULN) had a significant risk reduction when treated with high intensity statin versus low intensity statin.39 The current 2013 ACC/AHA Cholesterol guidelines recommend evaluating baseline assessment of liver enzymes but no routine follow up. These observational data support the safety of statins in patient with mild elevations of liver enzymes; however, if LFTs are elevated to >3 times the ULN, with no other explanation, the statin therapy should be discontinued.6,27
Statin-associated Hyperglycaemia
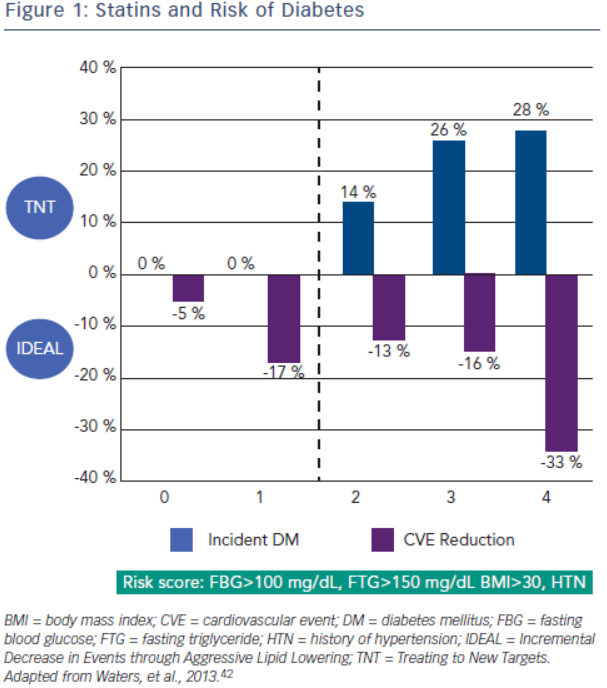
Increased incidence of diabetes is associated with statin use. A meta-analysis by Sattar et al., which included 91,140 patients found a 9 % increase in incident diabetes with statin use, and in another meta-analysis by Preiss et al., authors were able to show an increased risk with more intensive therapy.40,41 Risk factors for developing diabetes during statin therapy are fasting blood glucose >100 mg/ dL, fasting triglycerides >150 mg/dL, BMI >30 and hypertension. In a recent study, Waters et al. assessed the incidence of diabetes and cardiovascular event reduction from patients in the Treating to New Targets (TNT) and IDEAL studies. They concluded high intensity statins increased the risk of diabetes in patients with 2–4 risk factors but there was no increased risk in patient with 0–1 risk factors. However, in both groups statin therapy was associated with substantial risk reduction with the highest benefit in the patients with the most risk factors for developing diabetes (see Figure 2).42 Analysis from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), which randomised 17,603 patients without cardiovascular disease or diabetes to placebo versus rosuvastatin 20 mg demonstrated increased risk of incident diabetes in the statin group. Ridker et al. concluded overall benefit of statins on cardiovascular events and mortality exceeded the hazard of diabetes.43 A recent meta-analysis studied the effects of two genetic variants of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), the target enzyme inhibited by statins, on body weight and risk of diabetes. Authors also updated their previous meta-analysis on association of statins and diabetes. The study concluded that both genetic variants and statin treatment were associated with weight gain and diabetes.44
Conclusions
Statin use is expected to increase with the adoption of the new 2013 ACC/AHA Cholesterol guidelines, bringing new challenges for prescribers, with a focus on fixed-dose statins independent of baseline or achieved LDL-C levels. The intensity of statin dose is matched to a person’s baseline ASCVD risk. This is in contrast to the adapted EAS/ESC guidelines in Europe, which recommend tailoring therapy to target specific LDL-C levels. Statin intolerance is not uncommon and can be challenging to manage; however, several therapeutic strategies have been successful in achieving statin tolerance. Although statin use is associated with liver enzyme elevations and increased risk of incident diabetes, there continues to be a net benefit to statins. Individualised risk assessment and discussion of benefits and risks of statin therapy will likely lead to improved adherence and outcomes.