Takotsubo syndrome (TTS) is a transient form of acute heart failure that mimics an acute coronary syndrome (ACS), with comparable acute adverse outcome.1 Many hypotheses have been formulated, but the pathophysiology of TTS is still not fully understood. Recently, it was demonstrated that specific alterations in neurological response and sympathetic activation after emotional stimuli are present in TTS patients. These findings confirm the importance of brain–heart interaction in the development of this pathological process.2
Establishing Diagnosis
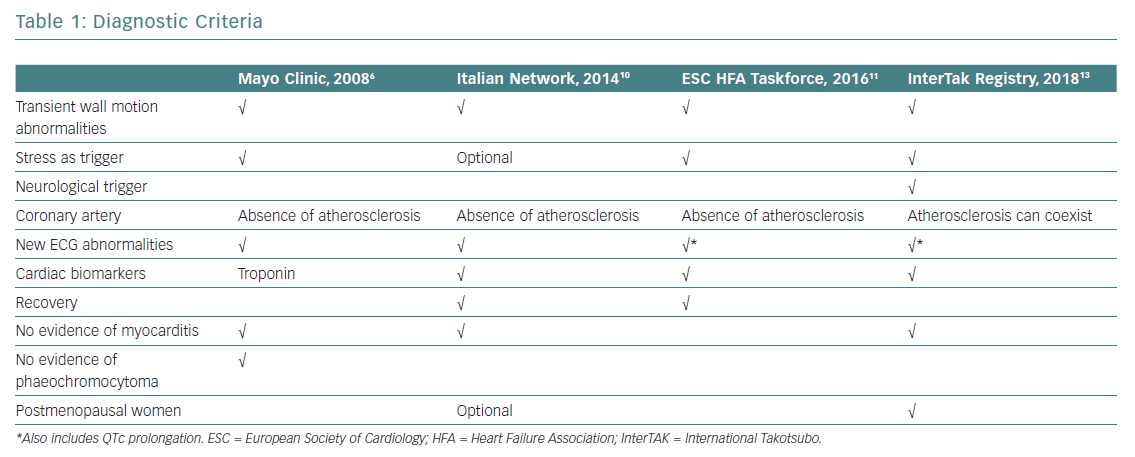
The evolution of diagnostic criteria in recent years reflects a renewed interest in TTS (Table 1). Despite these new developments, well-established, universally agreed TTS diagnostic criteria are still lacking.
The first diagnostic criteria for TTS were published by Japanese scholars of this condition in 2003, when TTS was still considered a relatively rare and somewhat mysterious entity more typical of Asia.3 Soon after, the Mayo Clinic group published their diagnostic criteria, which are still widely used today.4 Before then, TTS had been an obscure paragraph in small print at the bottom of the chapter on acute MI, considered for differential diagnosis and assumed to have excellent prognosis after the acute phase.
In 2006, the American College of Cardiology and American Heart Association defined TTS as a primary acquired cardiomyopathy,5 and this definition was rapidly adopted by the European Society of Cardiology (ESC). The initial paradigm that TTS meant a pseudoinfarction with normal coronary arteries was challenged by a revision of the Mayo Clinic Diagnostic Criteria in 2008.6 The authors of that revision highlighted the fact that obstructive bystander coronary lesions and classical ischaemic wall motion abnormalities in other territories are possible in TTS patients.6
Different criteria for TTS have been proposed by others, including Japanese Guidelines,the Gothenburg criteria, Johns Hopkins criteria, Tako-tsubo Italian Network proposal and the ESC Heart Failure Association (HFA) TTS Taskforce criteria, all of which have contributed to expanding and better specifying the pathognomonic features of a syndrome that was acknowledged to account for 2.2% of all cases of ACS.7–12
Based on experience obtained during development of the largest international TTS registry, the International Takotsubo (InterTAK) Registry, the following novel diagnostic criteria have recently been introduced:13
- TTS patients are characterised by transient acute left ventricular (LV) dysfunction (hypokinesia, akinesia or dyskinesia) that presents not only as the typical diffuse apical ballooning described in textbooks, but also involving the mid-ventricular and basal walls or causing only focal motion abnormalities. Right ventricular involvement can coexist. Patients with typical localised regional wall motion patterns can also transition to develop involvement of other segments. Regional wall motion abnormalities that extend beyond a single epicardial vascular distribution are considered an important feature distinguishing TTS from common ischaemic MI. Review of the large InterTAK Registry database showed that regional wall motion abnormalities in the myocardial territory subtended by a single coronary artery can also be present in TTS patients (focal TTS).
- An emotional, physical or combined trigger can precede a TTS event, but this is not obligatory.
- Neurological disorders (e.g. subarachnoid haemorrhage, stroke, transient ischaemic attack or seizures) and phaeochromocytoma may serve as triggers of TTS.
- New ECG abnormalities are very often present (not always the typical diffuse anterior ST-segment elevation, but also ST-segment elevation in different locations, ST-segment depression, T-wave inversion and prolongation of the QTc interval). Rare cases exist without any ECG changes.
- Levels of cardiac biomarkers (troponin and creatine kinase) are moderately elevated in most cases, often out of proportion with the severity and diffusion of the regional wall motion abnormalities.
- Significant elevation of B-type natriuretic peptide is common.
- Significant coronary artery disease (CAD) is not a contradiction in TTS.
- Infectious myocarditis must be excluded.
- Postmenopausal women are predominantly affected.
Of note, the InterTAK, Japanese and ESC HFA TTS Taskforce diagnostic criteria also include phaeochromocytoma as a specific cause of TTS, even if most other criteria mention phaeochromocytoma only with regard to differential diagnosis.7,11,13 Indeed, phaeochromocytoma can lead to a catecholamine storm with LV dysfunction, ECG abnormalities and increased biomarkers, as well as hypercontraction of sarcomeres and contraction band necrosis indistinguishable from TTS.
Based on LV functional changes most often observed in the initial echocardiographic or angiographic examination, TTS is classified into four different types: apical ballooning and three atypical types, namely midventricular, basal and focal wall motion patterns. The apical ballooning type is the most common, and occurs in 80% of TTS patients. It is characterised by hypo-, a- or dyskinesia of the midventricular and apical parts of the anterior, septal, inferior and lateral walls of the LV, associated with hyperkinesia of the basal segments. A peculiarity of apical TTS is the nipple type, described as a small to a very small region of preserved contractility in the most apical portion of an otherwise globally akinetic apex. When the region of preserved contractility is limited to the most apical portion of a globally a- to dyskinetic ventricular apex, it could be considered as a TTS variant of an extensive type of midventricular involvement or an early stage of mechanical recovery of classical apical ballooning. The midventricular type of TTS has been described in approximately 4–40% of patients with TTS. The basal type is rare, and only evident in 1–3% of all cases of TTS.
The presence of CAD should not be considered as an exclusion criterion; indeed, the prevalence of CAD in TTS patients is approximately 10%. In 1.5–7% of cases the wall motion abnormalities are limited to the distribution of a single coronary artery identifying a focal TTS type. In this situation, the differential diagnosis of TTS, ACS or myocarditis will ultimately require cardiac MRI demonstrating myocardial edema rather than late gadolinium enhancement in case of TTS.14
Predicting Outcome
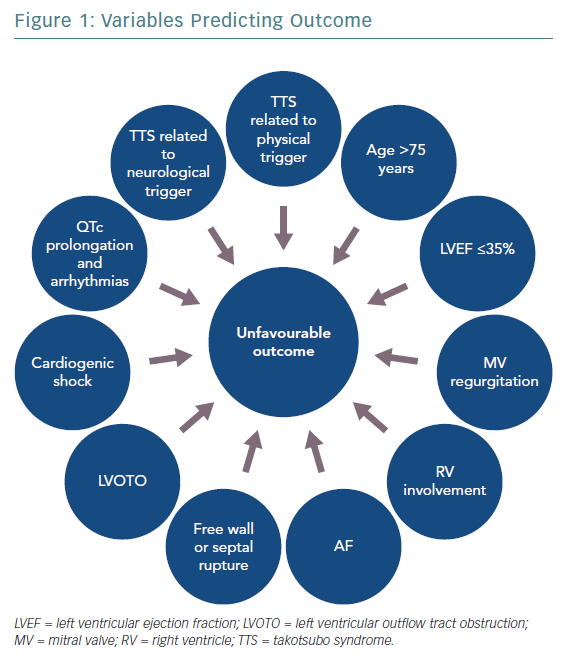
Figure 1 shows variables predicting outcome in TTS. The clinical features of TTS can vary widely and include life-threatening phenotypes. A new risk classification based on the trigger type, the InterTAK Classification, defined TTS secondary to neurological disease as the one associated with the worst short-term prognosis.15 TTS secondary to physical stressors has also shown higher long-term mortality compared with ACS, whereas a more favourable outcome was associated with emotional triggers. Hence, based on the triggering factor, TTS can either be a benign or life-threatening condition.
Many other variables have been proposed as markers of prognosis in TTS patients. Male sex and old age are associated with higher mortality and complication rates, probably due to the more frequent coexistence of underlying comorbidities.16,17 Among echocardiographic parameters, a low LV ejection fraction (LVEF) on admission has been demonstrated to be an independent predictor of mortality in TTS patients in terms of both in-hospital and long-term outcome.18,19 Patients with LVEF ≤35% were usually older, triggered by physical events and had a higher number of comorbidities. Indeed, it has been suggested that severe systolic dysfunction could identify a more vulnerable phenotype of TTS and that the apparent LVEF normalisation at follow-up still conceals persistent structural and functional abnormalities.19
In the acute phase, TTS patients may experience complications including LV mechanical complications (free wall or septal rupture), LV outflow tract obstruction (LVOTO), cardiogenic shock (CS), arrhythmias and thromboembolic events.
The presence of LVOTO, mitral valve regurgitation (MVR) and right ventricular involvement may be linked to higher CS rate, whereas a severely reduced LVEF has been correlated not only with CS, but also with increased risk of developing life-threatening arrhythmias.
Regarding CS, Di Vece et al.20 suggested that parameters easily detectable on admission, such as apical TTS, physical stress, lower LVEF, diabetes and AF, may identify patients at higher risk. AF was identified as a predictor of poor short- and long-term prognosis, probably because of the haemodynamic consequences of the loss of atrial contraction and the increased risk of thromboembolic events.21
Conversely, the morphological type of TTS and the coexistence of a CAD were not found to be related to the incidence of in-hospital complications.
Considering electrocardiographic signs, QTc interval prolongation seems to be associated with a higher risk of ventricular arrhythmias,22 especially polymorphic tachycardia, whereas the predictive role of ST-segment elevation is controversial.23
Delivering Treatment
There are no randomised clinical trials on TTS. The lack of solid evidence and the reversible nature of TTS justify a medical approach focused on supportive care and treating complications promptly until the patient has recovered.
Acute Phase Management
Once the presence of an ACS is ruled out, the acute management of TTS should be based on risk stratification, with aggressive monitoring for early detection and prompt treatment of complications in high-risk patients, reserving more conservative management for patients at low risk. In-hospital admission to a high-dependency unit or a ward capable of performing 48–72 hours of electrocardiographic monitoring is required for all patients.
Risk Stratification
Lyon et al. stratified lower- and higher-risk patients primarily on the basis of LVEF.11 In milder cases with an LVEF >45% and no complications, the patient can be discharged early from hospital.11 If LVEF is 35–45%, heart failure medications, including angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) and beta-blockers (BBs), should be considered.24 Diuretics or nitroglycerine can be used to reduce congestive symptoms.
Special attention should be paid to patients at risk of LVOTO, which can be worsened by vasodilators and may respond favourably to BBs. High-risk patients need intensive care, including invasive pressure lines to monitor arterial and central venous pressure. Serial echocardiographic examinations are mandatory for assessment of cardiac filling and ventricular function, as well as for the early identification of any mechanical complication, such as LVOTO.
Trigger Identification
Many emotional or physical triggers of TTS have been described, but in some cases no stressor can be identified. Because different prognostic effects have been observed for specific triggers,15 we recommend careful investigation for the potential stressor of TTS. Furthermore, if the trigger includes a medical condition causing persistent pain or discomfort and an elevated sympathetic drive, appropriate specific treatment should be rapidly initiated.
Management of Acute Complications
CS represents one of the leading causes of mortality in the acute phase of TTS. Because CS can result from a large adyskinetic area or be a consequence of hypercontractility of the basal segments leading to LVOTO, echocardiography is required to identify the mechanism and adapt treatment.
In patients with LVOTO, prevention of hypovolaemia with appropriate crystalloid infusion is indicated. Failure to detect the presence of LVOTO can lead to the use of drugs such as diuretics, vasodilators or inotropic agents to treat hypotension and lung congestion that can aggravate the obstruction and trigger a dangerous vicious circle.
Some studies suggest that BBs may reduce LVOTO by decreasing basal hypercontractility, increasing LV filling and reducing heart rate. However, randomised studies to confirm or contest short- and long-term usefulness of BBs in TTS management, and, if useful, which BB to use, are lacking and the evidence available is conflicting. In the Spanish REgistry for TAKOtsubo cardiomyopathy (RETAKO), the use of BB in patients with CS was associated with a lower 1-year all-cause mortality, whereas, Isogai et al. found no benefit in the early use of BB to reduce in-hospital mortality in TTS patients.25,26
Propranolol, esmolol and metoprolol can reduce LVOTO in TTS patients.27–30 Esmolol use may also have an additional advantage represented by its rapid onset and short duration of action. In any case, before starting BB therapy, the presence of phaeochromocytoma must be excluded.
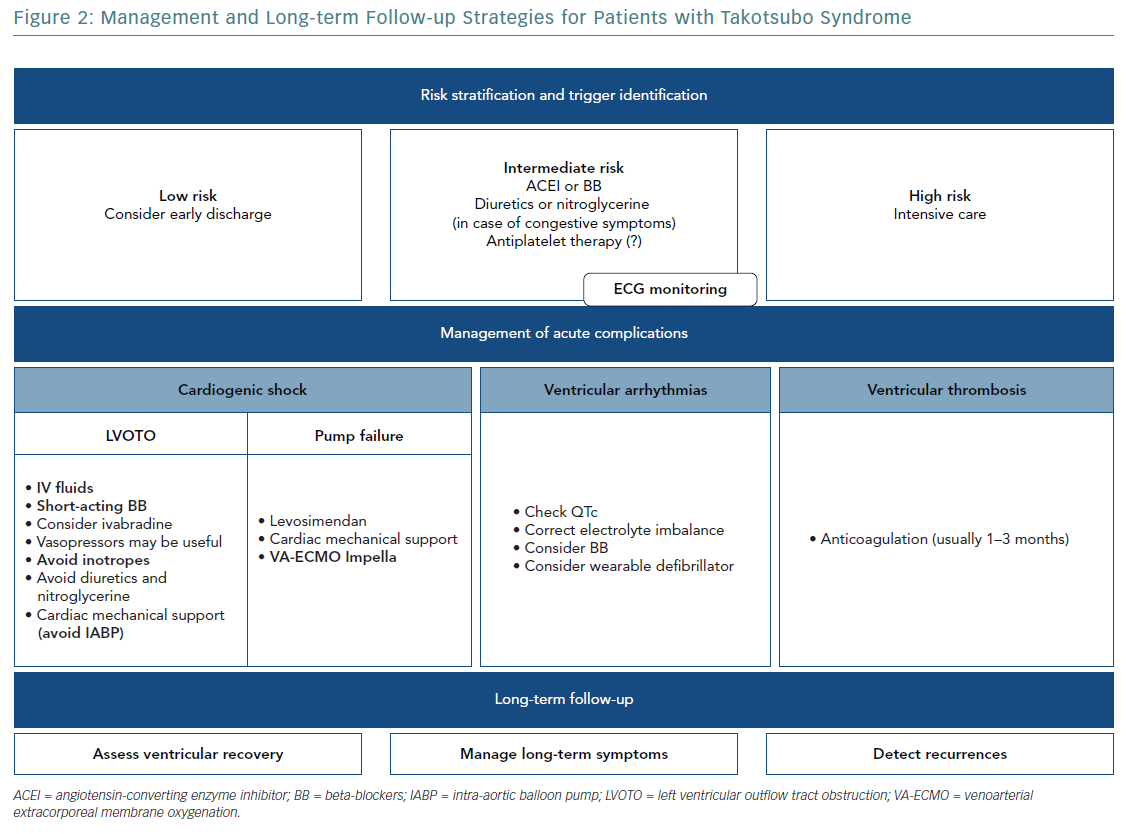
Ivabradine can reduce heart rate without altering myocardial contractility and could be an alternative to BBs, especially when tachycardia is associated with severely depressed myocardial function.31
In case of shock and concomitant LVOTO, peripherally acting vasopressor drugs (phenylephrine or vasopressin) may be useful because they increase blood pressure without worsening LVOTO.32
Inotropic agents, increasing basal segment contraction, may provoke or exacerbate LVOTO, worsening haemodynamic instability. Furthermore, given the presumptive role of catecholamines in the pathophysiology of TTS, the use of inotropic agents may have more deleterious consequences than possible benefit, so that the use of exogenous catecholamines should be discouraged. This recommendation is made stronger by recently published data on the reduced short- and long-term survival of TTS patients treated with catecholamines, although this evidence may be the result of a selection bias, because patients needing inotropic agents may represent a more severe TTS phenotype.33 Levosimendan can play an important role in the management of CS, improving cardiac function and reducing mortality.
The potential contraindications to most of the drugs used to support pressure in CS suggests an early consideration of mechanical support devices as a bridge to recovery. Treating TTS patients in CS with cardiac mechanical support, including intra-aortic balloon pumps (IABPs), such as Impella, and/or venoarterial extracorporeal membrane oxygenation (VA-ECMO), may improve their prognosis.20
Before considering IABPs, LVOTO must be first excluded because the reduction in afterload by IABPs may worsen obstruction and haemodynamic instability. However, based on results of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial, IABPs are not recommended as routine therapy for patients with CS.34 Lyon et al. suggested avoiding the use of IABPs in TTS patients.11 Some case reports indicate that Impella may represent a valid therapeutic option in TTS complicated by CS and LVOTO, potentially leading to more rapid LV function recovery and better clinical outcome.35 VA-ECMO has also been proposed for patients with refractory CS; its early role may be lifesaving in TTS patients who are not responding to conventional treatment, and especially in the presence of LVOTO.36
Continuous ECG monitoring at least for 24 hours is recommended in TTS patients because arrhythmic complications (ventricular arrhythmias, AF or conduction disorders) could occur.24
Polymorphic ventricular tachycardia (torsades de pointes) has been documented in association with QTc prolongation.37 Therefore, drugs acting on the QT interval, as well as catecholamines (because of their potential proarrhythmic effects), should be avoided and electrolyte imbalances should be checked and corrected.24 However, the correlation between a long QTc and ventricular arrhythmias is not universally endorsed, and monomorphic ventricular tachycardia has been described in patients with a normal QT interval, and is probably associated with an increased 1-year mortality rate.38 BBs may be useful in preventing ventricular arrhythmias and in case of a long QTc. However, BBs should be avoided in patients with bradycardia considering the risk of bradycardia-induced ventricular tachycardia.
As in ST-elevation MI patients, the presence of malignant ventricular arrhythmias in the acute phase of TTS does not require an ICD, with the rare exceptions of patients with severe persistent impaired LV function.
In this context, a wearable defibrillator (‘left jacket’) could be considered as a bridge or an alternative to an ICD to protect against life-threatening arrhythmias until LV function recovers and the risk of recurrence is assessed.
AF is the most frequent arrhythmic complication described in TTS, and it has been associated with worse clinical presentation.21
In case of complete atrioventricular block, the conduction disorder could persist beyond the acute and subacute phase of the disease, thus justifying permanent implantation of a pacemaker in these patients.
Hypercoagulable State
Apical ballooning with high troponin levels on admission was described as a marker of high thromboembolic risk.39 Thromboembolic complications may be associated with the catecholamine surge, endothelial dysfunction and blood stasis caused by the regional wall akinesia. Although it has been proposed that prophylactic oral anticoagulation be considered in high-risk patients, its role in the treatment of TTS remains to be determined.39 However, oral anticoagulation is recommended to prevent embolic episodes when a ventricular thrombus is detected, usually for 1–3 months and at least until the thrombus resolves and LV function recovers.11 Development of a thrombus in the right ventricle in patients with TTS has also been described, strengthening the usefulness of right ventricle assessment by transthoracic echocardiography.40
Considering that TTS could be triggered by neurological disease, bleeding risk should be assessed before starting anticoagulation, in addition to the potential risk of LV rupture.
The effects of catecholamines on platelet activation and endothelial dysfunction have been described, but there is still no agreement regarding antiplatelet therapy in TTS patients.41,42
Because TTS often mimics an ACS, many patients initially receive antithrombotic treatment, but once MI is excluded, it has been suggested to that P2Y12 receptor antagonists are withdrawn.11 Aspirin was found to be unrelated to any improvement in reducing the recurrence of TTS.43,44 In contrast, in a retrospective study, the use of aspirin, alone or in combination with clopidogrel, was described as an independent predictor of a lower incidence of cardiac events during hospitalisation.45 Because a benefit was found in short-term outcomes, TTS patients may be discharged with an indication to continue antiplatelet therapy for at least 2 months, especially if there is evidence of concomitant coronary atherosclerosis.24,46 The addition of BBs and ACEIs can also contribute to reducing mortality.
Long-term Management
In TTS patients, it is unclear whether heart failure therapies should be continued once the patient’s LV function has normalised. Although most patients recover rapidly after the acute episode, increasing evidence indicates that physiological abnormalities may persist, and some patients continue to suffer cardiac symptoms after the acute episode. Templin et al. showed that ACEIs or ARBs were associated with improved 1-year survival and a lower prevalence of recurrence.1
Recently, it was suggested that BBs are associated with lower major adverse cardiac events (cardiac death, heart failure, acute MI, TTS recurrence), especially in patients with an ejection fraction ≤35% at discharge.19 In the same study, ACEIs and ARBs were associated with fewer cardiac deaths at long-term follow-up, but had no effect on overall mortality.19 A meta-analysis has reported that BBs, ACEIs, ARBs, statins and aspirin have no benefit on mortality and recurrence in TTS patients.43
All TTS patients should be followed-up to assess complete ventricular recovery, manage long-term symptoms and detect recurrences. In addition, patients’ comorbidities should be carefully monitored because most of the long-term mortality in TTS seems related to coexisting non-cardiac conditions. Indeed, it has been suggested that the eventual neurological or psychiatric stressors are treated, because they are observed more frequently in the case of TTS recurrence.47
Recurrences can occur with different triggering factors and ballooning patterns. Moreover, it has been shown recently that beta1-adrenoceptor-selective antagonists are not useful in preventing recurrences.47 However, because there is no agreement regarding the long-term management of TTS, individualised treatment is often required (Figure 2).
Conclusion
TTS is an emerging condition associated with a heterogeneous clinical course, which ranges from a rapid, full recovery to poor early and long-term outcomes. Neither the physiopathological mechanisms underlying this disorder nor the risk for individual patients of developing adverse LV remodelling, heart failure or other complications are fully understood. The lack of rigorously tested treatment strategies for TTS patients represents an important limitation in guiding everyday clinical practice. Risk stratification is the future challenge to enable appropriate decisions to be made regarding the clinical management of TTS patients, in terms of both in-hospital and long-term outcomes, in order to offer better individualised patient care.