Heart failure (HF) affects more than 6 million people in the US and results in more than 1 million hospitalisations per year.1 In patients aged ≥65 years, there are more hospitalisations for a primary diagnosis of HF than any other condition.2 HF is a debilitating illness, associated with significant morbidity and mortality, rehospitalisation and societal costs.3 Current guidelines and position statements emphasise the management of patients with overt symptomatic disease, but the aging of the population and the increasing prevalence of congestive HF underscores the need for a shift towards effective prevention and management of patients with left ventricular (LV) dysfunction prior to the development of symptoms.
HF is considered a progressive disorder characterised by four stages:
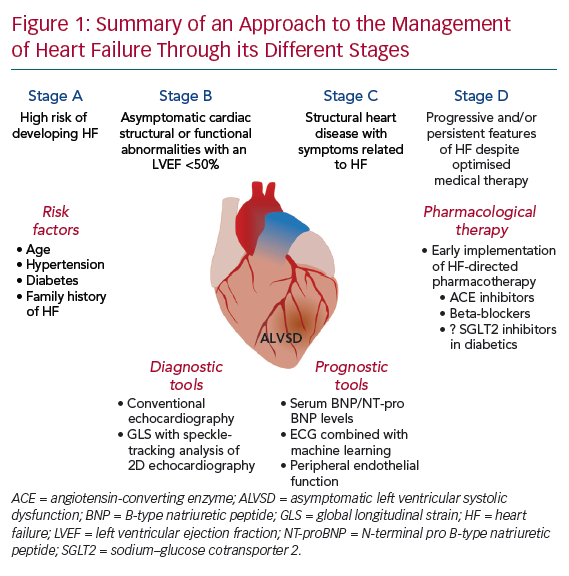
- Stage A, at high risk of developing HF;
- Stage B, structural heart disease without symptoms of HF; and
- Stage C/D, structural heart disease with symptoms related to HF.4
Asymptomatic LV systolic dysfunction (ALVSD), classified as stage B HF, is defined as depressed LV systolic function in the absence of clinical HF (Figure 1). The early initiation of therapies in patients with presumed ALVSD has been shown to lead to better outcomes.5,6 Nevertheless, there is considerable uncertainty surrounding the current definition of ALVSD, its prevalence and clinical importance and the clinical tools that may be of value in guiding management. In this article, we clarify these issues and highlight potential opportunities for future investigations to better address aspects of our understanding of this complex syndrome.
Prevalence and Prognosis of Asymptomatic Left Ventricular Systolic Dysfunction
In the Cardiovascular Health Study, echocardiography was performed in 5,649 subjects,7 7.3% of whom were classified as having ALVSD with an LV ejection fraction (EF) <55%.8 This was a population-based longitudinal study among adults aged ≥65 years with a history of coronary artery disease and stroke who were sampled from Medicare eligibility lists in predetermined geographic regions of the US. The study was undertaken in 1989 and advances in risk factor management and pharmacotherapy have changed the clinical profile of cardiovascular patients since then. Nevertheless, that study permitted evaluation of cardiovascular risk factors in older adults, as well as in particular groups that had previously been under-represented in epidemiological studies, such as women, which accounted for almost 50% of the Cardiovascular Health Study cohort.
In another population-based sample of 2,029 participants aged >45 years, 23% had stage B HF, characterised by asymptomatic cardiac structural or functional abnormalities with an LVEF <50%.9 Among patients with stage B HF, the risk of all-cause mortality was fourfold greater in men than in women after adjusting for age (p=0.01), and there was a tendency for an 1.8-fold increased risk of all-cause mortality for those with stage B HF after adjusting for age and sex compared with patient with stage A HF (p=0.08). Further, deterioration from stage B to stage C HF was associated with a significant increase in all-cause mortality (HR 9.6; 95% CI [6.8–13.6]; p<0.0001).9 That study was based on residents from Olmsted County (MN, US), which comprises >90% white people of northern European descent, representing a largely homogeneous and select racial group.
Further, observations from the Framingham Study revealed that subjects with ALVSD had a nearly fourfold increased risk of death than subjects with a normal LVEF >50%.10 In another study, compared with individuals with a normal LVEF (≥55%), ALVSD was associated with an increased risk of incident HF (HR 1.60; 95% CI [1.35–1.91]), cardiovascular mortality (HR 2.13; 95% CI [1.81–2.51]) and all-cause mortality (HR 1.46; 95% CI [1.29–1.64]), albeit with a lower risk than individuals with symptomatic LV systolic dysfunction.8 That study included patients with a mean age of 73.0 (SD ±5.6) years who were followed for a median duration of 11.7 years.
In addition, in a meta-analysis that included 11 studies evaluating 25,369 patients with ALVSD followed-up for a mean period of 7.9 years, the investigators found an adjusted relative risk of 4.6 for progression to overt HF.11 The risk of progression to overt HF was higher in patients with an LVEF <40% than in those with a mid-range LVEF of 40–49% (HR 7.8 vs. 3.3, respectively).10 These individuals are not only at risk of progressing to stage C/D HF, but are also at an increased risk of death. Further studies including younger and more diverse populations that also follow-up patients for longer periods of time could contribute more to our understanding of the natural history of ALVSD and the role played by conventional cardiovascular risk factors, including hypertension and diabetes, the presence of coronary artery disease and the implementation of cardiovascular pharmacotherapy. Nevertheless, there appears to be a significant association between ALVSD and conversion to clinically overt HF, as well as increased mortality, underscoring the importance of detecting ALVSD early.
Detection of ALVSD could allow for the early initiation of interventions such as pharmacological therapy to mitigate the progression of disease and improve outcomes in this group.11 However, at present routine echocardiography to screen for ALVSD is not recommended, and has not been shown to be cost-effective.12 Consequently, alternative cheaper, non-invasive tools that could assist in the identification of patients with or at risk of developing ALVSD could be of value.
Imaging Techniques to Assess Asymptomatic Left Ventricular Systolic Dysfunction
Congestive HF is often the end stage of progressive deterioration of LV function, which can remain asymptomatic for many years. In fact, ALVSD is considered to be as common in the general population as overt congestive HF.13 Numerous challenges arise when attempting to classify ALVSD as a homogeneous syndrome. In particular, studies defining ALVSD in patients have focused exclusively on LVEF, which can lead to a number of challenges.
First, studies have used multiple different cut-offs as their definition for impaired LVEF, ranging from more typically <50% to less frequently <35%, creating a heterogeneous group of individuals who are likely to have different phenotypes, risk for progression and prognosis despite having no symptoms. Although decreases in LVEF indeed correlate with worse clinical outcomes,14,15 the inverse relationship between LVEF and mortality plateaus at an LVEF of 40–45%, above which LVEF may not be directly related to mortality.15
Second, this definition focuses purely on systolic function, and moreover defines systolic function solely on the basis of LVEF, which represents only a single facet of ventricular function. This approach creates an arbitrary separation between systole and diastole, rather than evaluating cardiac function throughout the entirety of the cardiac cycle, and excludes the potential role of diastolic function, which, in itself, has prognostic value.16,17
Third, LVEF can change substantially depending on loading conditions of the LV, and thus does not necessarily reflect intrinsic myocardial contractile property.18 Fourth, although LVEF has remained a cornerstone of therapeutic decisions relevant to myocardial performance, it can remain normal despite the presence of significant LV dysfunction related to other coexisting factors, including LV hypertrophy and/or decreased cavity size, leading, in turn, to a reduced stroke volume.19 Thus, a definition limited to LVEF could overlook the broader evaluation of ventricular function.
The assessment of global longitudinal strain (GLS) from speckle-tracking analysis of 2D echocardiography has become a clinically feasible adjunct to LVEF for the assessment of myocardial function. In fact, GLS correlates with mortality independent of and incremental to LVEF in patients with HF with a reduced EF and following an acute MI.20–22 In a meta-analysis including 5,721 patients across 16 studies, the investigators showed that GLS was a stronger predictor than LVEF of all-cause mortality, as well as a composite of cardiac death, HF hospitalisation and malignant arrhythmias, and could, in fact, add incremental predictive value for mortality in individuals with an LVEF >35%.23 In addition, GLS has been proposed as the test of choice for monitoring asymptomatic cardiotoxicity related to chemotherapy, where impairments in GLS have been shown to precede and predict reductions in LVEF.24,25
Reduced myocardial deformational characteristics have also been demonstrated as the only sign of LV dysfunction in other groups at risk of HF. For example, in individuals with hypertension and a normal LVEF, independent of LV hypertrophy and diastolic dysfunction, reduced longitudinal strain confers an elevated cardiovascular risk.26,27 In asymptomatic individuals with diabetes, of whom up to one-third have an abnormal GLS with normal LVEF and diastolic function, GLS predicts worse outcomes in individuals with a normal LVEF.28–30 Further, in individuals with valvular heart disease, there is increasing evidence to suggest that GLS may be of independent predictive value over and above LVEF.31–33 Thus, although evaluating GLS may be technically challenging, its practical incorporation as a material index of myocardial function could enhance the ability to identify individuals with ALVSD across a range of cardiac diseases, and potentially with greater accuracy than currently used LVEF. Further studies are required to evaluate the potential utility of GLS in this regard.
Other issues related to identifying individuals with putative ALVSD include the fact that studies have relied largely on echocardiography, which has its own limitations with interobserver variability associated with differences in operator expertise and quality of image acquisition, as well as systemic limitations related to geometric assumptions and LV cavity border tracing. Alternative imaging modalities to identify ALVSD, such as cardiac MRI (CMR) and multigated acquisition scans, have featured less frequently in studies. Despite not carrying the same issues related to operator dependence, these techniques have their unique modality-specific limitations and provide measurements of LVEF that correlate differentially with those obtained using other imaging techniques. CMR in particular has been regarded as the gold standard to measure ventricular volumes and mass using a simple acquisition of a 2D stack of contiguous short-axis cines with full biventricular coverage, primarily due to its accuracy and reproducibility.34,35 GLS can be quantified using CMR, and correlates well with GLS measured by echocardiography.36 Although the clinical utility of CMR has developed rapidly as a consequence of remarkable advances in technology and imaging techniques, high cost and limited availability remain barriers to the widespread expansion and application of this modality when considering screening for ALVSD in the general population.
Thus, improved clarification of the definition of ALVSD in terms of which modality is used and which parameter of systolic and/or diastolic function is evaluated is important and holds additional important benefits. These benefits include:
- improving patient selection for clinical trials, which, going forward, could further enhance understanding of this syndrome;
- potential reclassification of individuals with stage B HF, which, in turn, could influence decisions regarding the optimal timing and frequency of consultation with specialist cardiology clinics;
- determining the nature and timing of pharmacotherapy with the view of preventing disease progression and reducing the risk of adverse events; and
- determining when invasive studies, such as coronary angiography, and invasive treatments, such as implantable cardiac device implantation, may be necessary.
Further studies are required to clarify the potential role of these interventions.
Non-imaging Tools to Assess Asymptomatic Left Ventricular Systolic Dysfunction
Individuals with ALVSD in whom treatment may be prognostically useful should be followed to assess clinical response and to identify those with disease progression. Moreover, clinical tools that offer an ‘abridged’ assessment(s) of cardiac function that could be used during follow-up, to assist with prognostication and to determine whether therapeutic goals are being met would be useful. To date, there have been a number of studies that have shown promising associations between non-invasive indices of cardiac function and ALVSD, which could potentially fulfil this role.
Serum B-type Natriuretic Peptide
Patients with ALVSD have evidence of secondary neurohormonal activation with higher concentrations of noradrenaline, atrial natriuretic peptide and B-type natriuretic peptide (BNP) than controls, but with levels that are lower than those in individuals with symptomatic HF.13,37–39 A previous study provided evidence of a significant association between elevated N-terminal pro BNP (NT-proBNP) concentrations and ALVSD in high-risk groups including those with diabetes or peripheral and cerebrovascular diseases.40,41 However, the largest community-based investigation using a BNP-based screening strategy to identify ALVSD yielded suboptimal diagnostic utility, with an area under the curve (AUC) of 0.72 in men and 0.56 in women.42
The recently published St Vincent’s Screening To Prevent Heart Failure Study (STOP-HF) randomised trial compared usual primary care against a BNP-based screening strategy among 1,374 participants with cardiovascular risk factors (mean age ± SD: 64.8 ± 10.2 years).43 In that study, the BNP-based screening strategy, using a cut-off value of 50 pg/ml, was associated with reduced combined rates of LV systolic dysfunction, diastolic dysfunction and clinically overt HF among individuals with stage A/B HF.43
Another prospective study involving diabetic patients (mean duration ± SD: 15 ± 12 years, mean HbA1c ± SD: 7.0 ± 1.1%) without known structural heart disease and an NT-proBNP cut-off value >125 pg/ml randomised individuals to either intensified treatment with aggressive up-titration of a renin–angiotensin system antagonist and beta-blocker therapy at a cardiac outpatient clinic or standard care at a diabetes care unit alone.44 Those in the intensified group had a reduced risk of hospitalisation and death due to cardiovascular disease at 2 years.44
Based on the results of these studies, the 2017 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America focused update of the 2013 ACC Foundation/AHA guideline for the management of heart failure gave class IIa recommendation on the use of a BNP/NT-proBNP-based screening strategy for individuals with stage A/B HF.4 Further studies are necessary to determine the cost-effectiveness of such an approach.
Electrocardiography
In a recently published paper, investigators from the Mayo Clinic reported that the application of artificial intelligence to the standard ECG was successfully able to identify individuals with LV dysfunction (LVEF ≤35%).45 After training a convolutional neural network using features from a resting 12-lead ECG in 44,959 patients, the investigators tested their application on an independent set of 52,870 patients and were able to identify individuals with an LVEF ≤35% when diagnosed with an echocardiogram with an AUC, sensitivity, specificity and accuracy of 0.93, 86.3%, 85.7% and 85.7%, respectively. Interestingly, patients who were misclassified as having an abnormal ECG by the artificial intelligence algorithm, despite having a normal LVEF (LVEF >50%) at baseline were found to have a fourfold increased risk of future LV impairment, defined as an LVEF ≤35%, than those with a negative ECG.45 An enhanced understanding of what specific abnormalities the algorithm identifies would be essential going forward. Further, how precisely the artificial intelligence-based application will become distributed on a population level and whether its detection algorithm will need to be calibrated in large-scale local training samples first before it can be clinically applied remain to be seen. It is likely that the algorithm will first need to undergo further validation in other populations, as well as refinement to detect milder, although nonetheless clinically relevant, LV dysfunction. Nevertheless, ECG-based screening offers promise in providing a ubiquitous and cost-effective tool to predict future deterioration of LV function in asymptomatic individuals. Identified individuals could then be encouraged to undergo echocardiography and/or could be advised to have more frequent clinical follow-up than may otherwise be indicated.
Non-invasive Tools to Measure Peripheral Endothelial Dysfunction
Endothelial dysfunction precedes atherosclerosis and independently leads to adverse cardiovascular events.46 Endothelial dysfunction can be measured peripherally and non-invasively using various tools, including flow-dependent hyperaemia with ultrasound and reactive hyperaemia-peripheral arterial tonometry (RH-PAT), using devices such as EndoPAT (Itamar Medical Ltd, Caesarea, Israel). The association between peripheral endothelial dysfunction (PED) and overt HF has been shown in several studies and supports the notion of vascular–cardiac coupling. In one study following 362 patients with HF and a reduced LVEF for 3 years, a significant association was found between PED and HF-related events, including the composite of cardiovascular death and HF hospitalisation.47 In another study, the authors found that baseline PED predicted HF-related hospitalisation in patients who had implanted cardiac resynchronisation therapy for HF.48 However, the association between PED and ALVSD has not been previously investigated.
Regardless of the presence of CAD, HF is associated with endothelial dysfunction due to reduced levels of synthesis, release and/or response to nitric oxide (NO).49–51 Impaired NO-mediated vasodilatory reserve contributes to exercise intolerance by increasing LV afterload and abnormal skeletal muscle signalling.52,53 In mice, a lack of endothelium-derived NO signalling may be associated with reduced capillary density in cardiac muscle through insufficient activation of vascular endothelial growth factor, leading to systolic dysfunction.54 Impaired endothelium-mediated vasodilation in HF is a generalised abnormality that occurs in both the peripheral and coronary circulation.55
Previously, we demonstrated that coronary microvascular endothelial dysfunction is present in patients with ALVSD.56 Another study showed that impaired endothelial-dependent vasodilation, measured using forearm blood flow in response to intra-arterial methacholine, was present and near maximal in individuals with mild HF (New York Heart Association class I and II), further evidence that endothelial dysfunction may be an early finding in HF.57 Thus, given the central role of NO bioavailability and activity in the assessment of PED using indices such as RH-PAT, for example, growing evidence suggests that the effects of impaired endothelial-derived NO contribute to the pathophysiology of LV systolic dysfunction and the progression of HF from its early stages.58 Thus, these patients may benefit from the initiation of therapy targeted at the NO pathway to address endothelial dysfunction and, in turn, potentially mitigate the progression to overt HF. The precise relationship between PED and ALVSD needs to be clarified with large prospective trials, and only then can the potential utility of measuring peripheral endothelial function as a screening tool for ALSVD be determined.
Management of Asymptomatic Left Ventricular Systolic Dysfunction
Identifying HF before the onset of symptoms could enable the implementation of therapy at a point along the natural history of the disease that may slow or terminate progression. Indeed, detecting subclinical LV impairment as a surrogate of future development of HF could form a useful clinical model to guide clinicians on the optimal timing of initiating therapy. To date, a screening process to identify individuals with ALVSD has not been endorsed, and further studies evaluating risk–benefit ratios with regard to treatment efficacy and cost implications are required.59
Nevertheless, among the limited data available to address the potential utility of intervening with treatment in patients with ALVSD, the Studies of Left Ventricular Dysfunction (SOLVD) prevention trial showed that the use of enalapril in patients with ALVSD brings about a significant improvement in mortality and morbidity.5 The Trandolapril Cardiac Evaluation (TRACE) trial also showed that the use of trandolapril reduced the risk of mortality in patients with a reduced LVEF (<35%) after MI.60 With further stratification based on patient symptoms, the use of trandolapril was also associated with a significant reduction in mortality, even in patients with ALVSD.60 Among patients with ALVSD, both the SOLVD prevention trial and the Survival and Ventricular Enlargement (SAVE) trial showed that administration of beta-blockers in addition to angiotensin-converting enzyme inhibitors reduced mortality and hospitalisation.1,6 The roles of other HF medication, including digoxin, aldosterone antagonists and direct renin inhibitors, have not been evaluated in patients with ALVSD.
Thus, the SAVE and SOLVD trials both demonstrated that early pharmacological treatment for ALVSD is effective. However, these studies are dated and defined LV impairment as an LVEF of <35–40%. There is a lack of evidence regarding early pharmacological intervention for ALVSD with an LVEF of 40–49%.
The Prospective Comparison of ARNi (angiotensin receptor–neprilysin inhibitor) with ARB (angiotensin receptor blocker) Global Outcomes in Heart Failure with Preserved Ejection Fraction (PARAGON-HF) trial, in which the effect of the ARNi sacubitril in combination with valsartan was tested for patients with stage C/D HF and an LVEF >45%, failed to show a reduction in HF hospitalisation and cardiovascular death.61 However, lower LVEF was associated with a reduction in HF hospitalisation and cardiovascular death in this population, indicating the potential benefit of sacubitril–valsartan in patients with more severe LVEF reduction.61 Further trials, including contemporary populations and therapeutics, are required to determine the best approaches to managing patients with ALVSD.
A useful approach would be to compare early intervention(s) against clinical surveillance, including potentially more frequent follow-up than may be otherwise indicated, without the implementation of additional therapy, to determine the most cost-effective approach to managing these individuals. Previously, clinicians considered pharmacotherapy for abbreviated periods of time, guided either by prognostication tools and/or serial clinical evaluation, or for predetermined time intervals in a bid to achieve an acceptable balance between risk mitigation and harms from undue medical therapy. However, the Therapy withdrawal in REcovered Dilated cardiomyopathy – Heart Failure (TRED-HF) trial demonstrated that withdrawal of pharmacological therapy in patients with dilated cardiomyopathy after recovery of HF symptoms and LVEF >50% led to relapse of HF in 40%, suggesting the need of lifelong treatment.62 Thus, at present, no data justify the withdrawal of pharmacological therapy for patients with recovered ALVSD.
The role of sodium–glucose cotransporter 2 inhibitors in this population needs investigation because these medications have shown a significant reduction in rates of HF hospitalisation in diabetic patients, including those with no known HF, and also in diabetic patients with multiple cardiovascular risk factors but without established cardiovascular disease or HF.63–65 Recently, the Dapagliflozin and Prevention of Adverse outcomes in Heart Failure (DAPA-HF) trial showed a significant reduction of mortality and HF hospitalisation even in non-diabetic patients, although only individuals with symptomatic LV systolic impairment were included in that study.66 Nevertheless, the study highlighted the potentially promising effects of SGLT2 inhibitors in patients with HF regardless of the presence of diabetes. Further studies regarding the effects of SGLT2 inhibitors on the natural history of ALVSD in patients with and without diabetes are needed to clarify their role in these groups.
Identifying ALVSD could facilitate the early initiation of cardioprotective therapy, which could contribute to efforts in reversing the widespread HF epidemic. Nevertheless, ongoing consideration must be given to resource allocation, distribution of specialist medical centres and the number and timing of patient visits that may be required for the large number of individuals in the wider community deemed to be ‘at risk’ but who have no conventional indications for imaging. This, in turn, will require wider health economic considerations, as well as those pertaining to clinical decision making alluded to above, when determining the optimal way to evaluate and manage patients in the early phases of the HF process.
Conclusion
ALVSD is common, and is associated with a significantly increased risk of progression to overt HF and death. A definition of ALVSD remains elusive secondary to challenges posed by heterogeneity of descriptions used in the literature coupled with heterogeneity in the non-invasive techniques used to study the phenomenon. These uncertainties underpin some of the knowledge gaps related to contemporary appraisal of ventricular function. Addressing these issues is integral to translating the identification of subclinical ventricular dysfunction into an opportunity to intervene with the potential to affect clinical outcomes. Screening asymptomatic individuals with echocardiography or CMR is currently not recommended, nor is it cost effective, and how these individuals may be recognised in the first instance remains undetermined. Non-invasive screening tools such as ECG evaluation with machine learning, laboratory assessment with serum BNP concentrations and measurements of PED offer promise in this regard, and could assist with prognostication, but each carries a number of caveats that will need to be addressed. Finally, individuals with ALVSD may potentially benefit from the early implementation of HF-directed pharmacotherapy, although the precise nature and timing of these approaches will need to be clarified in larger prospective studies using contemporary populations.