Cardiovascular disease (CVD) is the leading cause of mortality in the world, accounting for 17.9 million deaths per year and 31% of deaths worldwide.1 In addition to the toll on human life, the healthcare cost of CVD continues to grow, with estimates of up to US$1.1 trillion by 2035.1 Given the significant public health burden of disease as well as the cost of healthcare, we must shift our focus from downstream treatment to upstream prevention of CVD.
Guidelines on the prevention of CVD in clinical practice recommend the assessment of total CVD risk. Many risk assessment tools have been developed and validated, including the Systematic Coronary Risk Estimation (SCORE) system and Pooled Cohort Equations (PCE), which represent the gold standard in Europe and the US respectively.2,3 These tools incorporate age and gender, among other factors, to calculate an estimated risk of CVD over time. However, these risk assessment tools have inherent limitations, with several studies demonstrating over- or under-estimation of risk in certain populations, highlighting their imprecision.4–8
Total cholesterol, high-density lipoprotein (HDL) cholesterol (HDL-C) and low-density lipoprotein (LDL) cholesterol (LDL-C) are important parameters in determining CVD risk, though the standard lipid profile alone does not reliably capture all lipid-related atherosclerotic risk in an individual patient. Several other lipid and lipoprotein assays have been developed with the goal of guiding lipid-modifying therapies, to improve risk assessment and prevent incident or recurrent CVD.
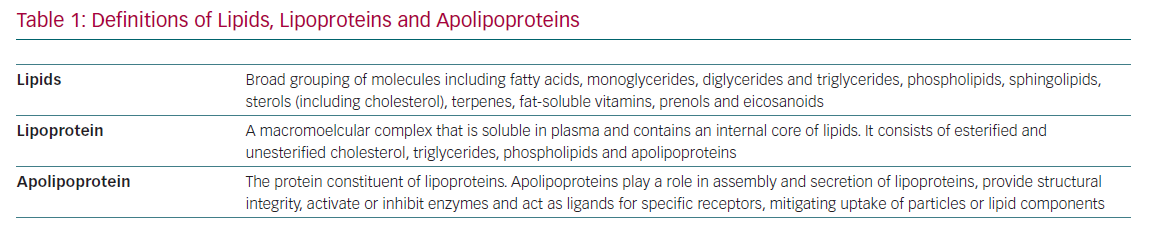
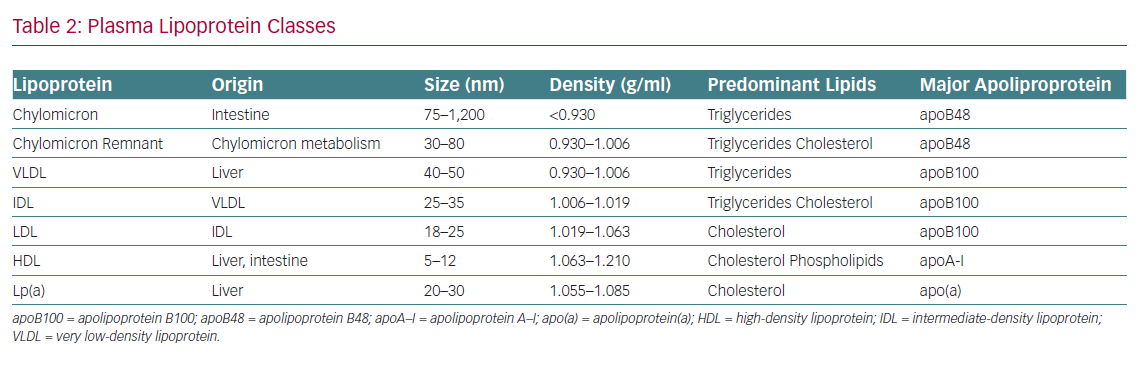
A fundamental understanding of terminology and basic lipoprotein physiology must be established in order to appropriately identify and implement these biomarkers of CVD risk (Tables 1 and 2). This article addresses the current state of the science regarding advanced lipid testing and its implications for clinical care.
Non-high-density Lipoprotein Cholesterol
Non-HDL-C represents the cholesterol contained in all lipoproteins except HDL-C and it can be calculated from the standard lipid panel by subtracting HDL-C from total cholesterol. It represents the cholesterol content present in all atherogenic lipoproteins and serves as a better surrogate for the overall atherogenic burden than LDL-C alone, making it a useful marker in the assessment of CVD risk.9
As non-HDL-C serves as a surrogate for the entire spectrum of atherogenic lipoproteins, estimation of lipoprotein-related atherosclerotic risk may be more accurate than simply using LDL-C.10 Moreover, non-HDL-C offers several additional advantages over LDL-C in assessing risk. For example, non-HDL-C is easily calculated from the standard lipid profile and incurs no added cost. It can be measured in the non-fasting state, making it easier to attain for the patient and the healthcare provider, although some guidelines suggest non-fasting lipid values are also acceptable.11 Non-HDL-C levels help to identify a subset of patients with residual CVD risk despite having controlled LDL-C, particularly in those with metabolic syndrome and/or diabetes.12,13
Several key organisations provide formal guidance regarding the clinical use of non-HDL-C. The 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guideline recommends using non-HDL-C as part of routine lipid analysis for risk evaluation in patients with diabetes or elevated triglycerides and in patients with very low LDL-C levels. They propose non-HDL-C targets of <2.2 mmol/l (<85 mg/dl), <2.6 mmol/l (<100 mg/dl), and <3.3 mmol/l (130 mg/dl) for people at very high, high and moderate risk, respectively.11 These targets are also referenced in a consensus statement released from the EAS and the European Federation of Clinical Chemistry and Laboratory Medicine (EAS/EFLM) as secondary treatment goals.14 The National Lipid Association (NLA) states that non-HDL-C outperforms LDL-C in the prediction of CVD, and thus advocates for its inclusion when reporting standard lipid laboratory values in a patient’s medical record.15 The 2018 American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guideline also mentions non-HDL-C in several capacities. According to the ACC/AHA guideline, non-HDL-C can be used to define primary hypercholesterolaemia (non-HDL-C 4.9–5.7 mmol/l; 190–219 mg/dl) as a risk-enhancing factor and may facilitate decisions regarding initiation of a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (non-HDL-C ≥2.6 mmol/l) (≥100 mg/dl) in those with established atherosclerotic CVD.16
Apolipoprotein B
Apolipoprotein B (ApoB) is a large surface protein present on atherogenic lipoproteins and serves as a macromolecular scaffold to provide structural integrity. It also serves as a ligand for the LDL receptor, which facilitates its clearance from the plasma. There are two major isoforms of apoB: apoB48, found on intestinally derived lipoproteins (chylomicrons and their remnants) and apoB100, found on hepatically derived lipoproteins – very LDL, intermediate-density lipoprotein, LDL and lipoprotein (a) (Lp[a]). Each of these atherogenic particles harbours a single copy of apoB. Thus, apoB represents a better proxy of total atherogenic lipoprotein particle concentration than the lipid fractions measured in the standard lipid panel.
To be clear, while both apoB and non-HDL-C are useful biomarkers for risk assessment, they quantify different parameters. ApoB represents the concentration of atherogenic particles in the plasma, whereas non-HDL-C represents the concentration of cholesterol trafficked by atherogenic lipoproteins in the plasma. However, non-HDL-C and apoB are highly correlated and both perform better than LDL-C when assessing risk of atherosclerotic CVD.17–19 While some studies have found apoB to be a superior biomarker of atherosclerotic CVD risk compared with LDL-C or non-HDL-C, others report similar risk prediction compared withnon-HDL-C.20,21 Measurement of apoB can be accomplished either directly or indirectly by vertical auto profile, nuclear magnetic resonance (NMR) or immunoassay.22 While all three methods are considered comparable by international standards, there is substantial variability in apoB measurement among these tests, with apoB levels found to be highest when measured by immunoassay, lower by NMR and lowest by vertical auto profile.22–24
The 2019 ESC/EAS guideline states that measurement of apoB should be performed as part of routine CVD risk evaluation in patients with diabetes or elevated triglycerides and in patients with very low LDL-C levels. ApoB is the preferred biomarker to guide cardiovascular risk management with on-treatment target levels of <1.2 µmol/l (<65 mg/dl), <1.6 µmol/l (<80 mg/dl) and <1.9 µmol/l (<100 mg/dl) in people considered very high, high, and moderate risk, respectively.11 The EAS/EFLM consensus statement mentions the measurement of apoB can be useful in those with a moderate estimated risk and additional metabolic risk factors.14 The NLA endorses measurement of apoB to guide risk assessment and to adjudicate the efficacy of lipid-lowering therapy in those at intermediate risk, in those with a strong family history of premature CVD, or in those with recurrent atherosclerotic events.25 The NLA also states that apoB measurement may inform the need to intensify lipid-lowering therapy, especially when apoB levels remain high despite attainment of LDL-C goals. The 2018 ACC/AHA cholesterol guideline mentions that apoB levels may be useful in identifying whether hypertriglyceridaemia is associated with increased atherosclerotic risk. There is considerable evidence that CVD risk is higher in those with hypertriglyceridemia and high apoB versus those with hypertriglyceridaemia and normal apoB levels.26–28 Therefore, when triglycerides exceed 200 mg/dl, apoB can be considered a risk-enhancing factor when its levels exceed 2.5 µmol/l (130 mg/dl).16
Low-density Lipoprotein Particle Number
LDL particle (LDL-P) number represents an alternative to LDL-C as a marker of CVD risk. While LDL-P represents the concentration in nanomoles of LDL particles per litre of plasma volume, LDL-C represents the cholesterol mass in milligrams found in LDL particles in a decilitre of plasma. Though related, the amount of cholesterol carried by LDL particles differs in and across individuals, with significant variability observed in numerous studies.29,30 The heterogeneity in cholesterol cargo among LDL particles leads to frequent discordance between concentrations of LDL-C and LDL-P. This observation is particularly evident in patients with low HDL-C, hypertriglyceridaemia, metabolic syndrome and diabetes.31–34 A study by Cromwell et al. was conducted to determine which of several measurements of LDL-related risk was most strongly related to incident CVD, and found that LDL-P was a more sensitive indicator of low CVD risk compared with LDL-C and non-HDL-C.35 Another study using data from the Multi-Ethnic Study of Atherosclerosis found that LDL-P was more closely associated with incident subclinical atherosclerosis compared with non-HDL-C.36
About 90% of apoB is carried on LDL in the fasting state.37 Thus, comparisons between LDL-P and apoB have been made to determine if discordance exists between these two closely correlated parameters. A meta-analysis of 25 clinical trials compared the performance of LDL-P and apoB to predict CVD events.38 The American Association for Clinical Chemistry Lipoproteins and Vascular Diseases Division Working Group on Best Practices found a strong association between apoB and LDL-P concentration with CVD events and concluded that both markers were largely comparable in their association with outcomes. A commentary by Master et al. echoed these findings, stating that either LDL-P concentration or apoB may be better predictors of CVD risk than the classic measurement of LDL-C. Thus, either marker can be incorporated into clinical practice when making decisions regarding initiation or intensification of lipid-lowering therapy.39
There is no mention of LDL-P measurement in the 2019 ESC/EAS guideline or 2018 ACC/AHA guideline when assessing CVD risk. The NLA states that clinicians can consider measuring LDL-P as an alternative to apoB.40
Lipoprotein(a)
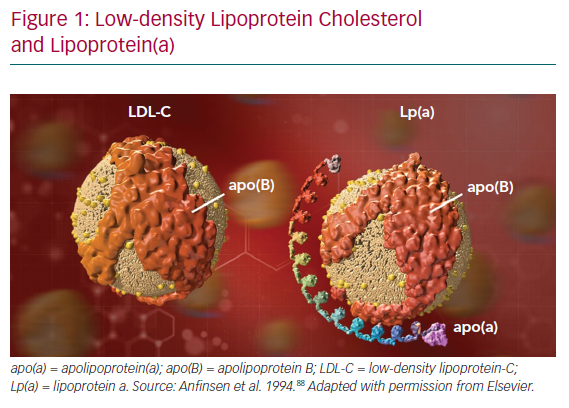
Lp(a) consists of a molecule of apolipoprotein(a) – apo(a) – a non-functional mimic of plasminogen, covalently bound to apoB on an LDL-like particle (Figure 1).41 Significant heterogeneity between apo(a) isoforms confers heterogeneity in Lp(a) particles. Plasma concentration of Lp(a) is >90% genetically determined in an autosomal co-dominant fashion, with adult levels achieved by about 5 years of age.42 Additionally, Lp(a) levels remain stable throughout life regardless of lifestyle. Interestingly, there is a strong established link between Lp(a) and calcific aortic valve stenosis (CAVS) though the mechanism remains unclear.43,44
High-quality evidence supports the relationship between Lp(a) and important CVD-related outcomes. Several observational studies, large scale meta-analyses, Mendelian randomisation analyses and genome-wide association studies suggest a likely causal relationship between circulating Lp(a) and MI, peripheral arterial disease, ischaemic stroke, heart failure, CAVS, cardiovascular mortality and all-cause mortality.45–48
Additionally, Lp(a) demonstrates an incremental predictive value that is additive to other traditional risk factors for CVD independent of LDL-C, non-HDL-C and other CVD risk factors.41,46,47 Unfortunately, methodologies of Lp(a) measurement are not standardised. Assays report results in either mass (mg/dl) or concentration (nmol/l) and direct conversion between the two units is not possible due to the variability among different apo(a) isoforms. Therefore, isoform-independent assays are necessary to avoid erroneous estimation of Lp(a) levels. The absence of evidence-based Lp(a) cut points in different risk groups, ethnic populations and comorbidities also limits its use on a large scale.
The 2019 ESC/EAS guideline suggests measurement of Lp(a) at least once in each individual’s lifetime to identify people with high levels, signifying a very high lifetime risk of CVD. People with very high Lp(a) can have a lifetime risk of atherosclerotic CVD equivalent to the lifetime risk of CVD observed in people with heterozygous familial hypercholesterolaemia, highlighting the need for early recognition and aggressive management.11,49 The authors of this guideline also recommend consideration of Lp(a) measurement in people with a moderate to high 10-year risk of atherosclerotic CVD. Similarly, the EAS/EFLM consensus statement mentions Lp(a) can be measured to help refine CVD risk and/or characterise dyslipidaemia when unclear.14 The NLA states it is reasonable to measure Lp(a) to assess atherosclerotic CVD risk in patients with a strong family history of premature CVD or recurrent cardiovascular events. However, they give a weaker recommendation for its use to aid in clinical decision making, stating it can be ‘considered for selected patients’.25 The 2018 AHA/ACC cholesterol guideline considers a Lp(a) ≥125 nmol/l (≥50 mg/dl) as a risk-enhancing factor, and its measurement can be considered in patients with a strong family history of premature CVD or personal history of CVD not explained by other traditional risk factors.16 Moreover, Lp(a) measurement should be considered in people with familial hypercholesterolaemia, given evidence that this condition and Lp(a) are synergistic in predicting early onset CVD and its severity.50
Several classes of therapeutics demonstrate the ability to lower Lp(a), including PCSK9 inhibitors, niacin, mipomersen, lomitapide, cholesterol ester transfer protein inhibitors and oestrogen, though clinical implications remain unclear.41,51–53 A novel antisense oligonucleotide that effectively reduces translation of APOA1 mRNA (APOA1 mRNA undergoes translation to become apolipoprotein A-I [apoA-I] protein) and plasma Lp(a) by about 80% is currently under development. Lipoprotein apheresis is an effective method for lowering plasma Lp(a) and remains an option in patients with progressive CVD despite optimal control of all other risk factors. Apheresis sessions are usually performed once every 2 weeks with sessions lasting 1.5–4 hours. In general, Lp(a) levels decrease acutely by 60–75% with each apheresis session, dependent upon baseline Lp(a) concentration and apheresis interval.54–56
Apolipoprotein A-I
ApoA-I is the major protein constituent on HDL and plays a central role in reverse cholesterol transport by stabilising the HDL particle, interacting with the ATP-binding cassette transporter I, activating lecithin cholesterol acyl transferase and acting as a ligand for the hepatic scavenger receptor.57–59 Levels of apoA-I are strongly correlated with HDL-C, with evidence suggesting apoA-I gene expression may be responsible for determining plasma HDL concentrations via changes in clearance rate.60,61 However, the stoichiometry of apoA-I differs from apoB in that more than one molecule of apoA-I can be present on an individual HDL particle. As such, apoA-I cannot serve as a reliable proxy for HDL particle concentration compared with apoB which can serve as an excellent surrogate of atherogenic particle concentration.
The Bogalusa Heart Study played a pivotal role in establishing the link between apoA-I and CVD by demonstrating that children of parents with a history of CVD had low apoA-I levels.62 Other studies went on to strengthen this association by establishing that baseline levels of HDL-C and apoA-I can predict MI independent of other coronary risk factors (including lipids) and are associated with an increased risk of total and cardiovascular mortality.63,64 However, when accounting for apoA-I independent from HDL-C, this biomarker seems to lose its predictive ability for CVD events.65,66 Some experts believe that the ratio of apoB/apoA-I (or atherogenic particles/anti-atherogenic particles) has significant value in predicting CVD risk, though results from the literature are inconsistent. For example, data from the Apolipoprotein-Related Mortality Risk (AMORIS) trial demonstrated that apoB/apoA-I was superior to total cholesterol/HDL-C ratio in predicting CVD events, while data from the Framingham Offspring Study demonstrated that these two ratios were comparable in their ability to predict CVD events.67,68 Neither the 2019 ESC/EAS guideline, 2018 AHA/ACC, guideline, nor the NLA provide guidance on the clinical use of apoA-I in assessing CVD risk.
High-density Lipoprotein Particle Number
HDL particles are heterogeneous in composition, structure, metabolism and function, leading to differential effects on atherosclerosis.69 Akin to alternative measurements of LDL, HDL particle measurement represents the concentration of HDL particles within a given volume of plasma, whereas HDL-C represents the mass of cholesterol carried by HDL particles in a given volume of plasma. Both HDL particle number (HDL-P) and HDL-C are independently associated with CVD risk.70 Measurement of HDL-P is accomplished by NMR or ion mobility analysis, with most studies using NMR. In general, HDL particles are thought to enhance vascular health by promoting cholesterol efflux, endothelial integrity, antiplatelet activity and anticoagulation.71,72 However, a direct mechanistic relationship between HDL-P and CVD has not been fully elucidated.
Several studies have compared the ability of HDL-P and HDL-C to predict CVD events, with the majority demonstrating that HDL-P performs as well or better than HDL-C.70,73–78 Notably, the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) found that HDL-C did not predict CVD after adjusting for HDL-P, while HDL-P remained significantly and inversely associated with CVD after adjusting for HDL-C.75,76,79 Furthermore, several studies assessing HDL particle size report that patients with CVD tend to have more small compared with large HDL particles, with larger particles mediating atheroprotection.80–82 Conversely, other studies have shown the opposite.83 Such discrepancies in the data have made interpretation difficult.
Currently, there are no guidelines that recommend the use of HDL-P to assess CVD risk. The NLA does not recommend measuring HDL-P and discourages using HDL-C as a target for lipid pharmacotherapy.40
High-density Lipoprotein Subfractions
NMR technology and ultracentrifugation enable scientists and researchers to further classify HDL-P into subfractions, HDL2 (large, buoyant HDL) and HDL3 (small, dense, protein-rich HDL). While there does seem to be an association between HDL subfractions and CVD, many studies are conflicting due to differences in study design, patient population, adjustment of confounders, the technique used for HDL subfractionation and the different studied outcomes.84
A review of the literature by Superko et al. was conducted to better understand the clinical utility of HDL subfractions. Eighty studies were evaluated to assess the ability of HDL2 and HDL3 to predict CVD and found that neither HDL subfraction consistently improved identification of individuals at risk.85 Of the eight prospective studies evaluated, four demonstrated an association between both subfractions, three demonstrated an association with HDL3 alone and one demonstrated an association with HDL2 alone. In an attempt to harmonise the conflicting data on HDL subfractions, a consensus statement by Rosenson et al. proposed a new classification of HDL based on the various fractionating methods.86 Five distinct subfractions were proposed – very large, large, medium, small, and very small – based predominantly on size and density.87 However, given conflicting data, cost and difficulty in measurement, HDL subfraction measurement is not recommended for clinical CVD risk assessment. The ESC/EAS, ACC/AHA and NLA do not support the measurement of HDL subfractions.
Conclusion
Advanced lipid testing encompasses a wide range of diagnostic laboratory tests as illustrated in this article. Selective use of lipid and lipoprotein biomarkers enhance prediction of CVD risk in patients whose risk is difficult to discern and helps the assessment of the efficacy of lipid-lowering therapy. Further studies are warranted to better understand the usefulness of these risk biomarkers. Additionally, variability of assay methodology and reporting also serve as a barrier for widespread clinical implementation. As of now, the most promising markers are non-HDL-C, apoB, and Lp(a) based on the quality and consistency of the literature. When used in the appropriate context, they can provide incremental prognostic information, enhance shared decision-making and inform therapeutic decisions to improve cardiovascular health.