Transcatheter aortic valve implantation (TAVI) is the therapy of choice for patients with symptomatic severe aortic stenosis who are unsuitable for surgical aortic valve replacement and for elderly patients with high operative risk.1,2 It is also considered to be a more than valid and reasonable alternative to surgical aortic valve replacement for patients with moderate to high surgical risk.3,4 Of note, two recently published trials assessing TAVI in low-risk patients have shown that TAVI is at least non-inferior and is likely to be superior to surgery in major outcomes in this scenario.5,6 Overall, these findings suggest that indications for TAVI will be expanded and the number of patients treated with this technique will continue to grow exponentially in the near future.
Improvements in patient assessment, new generation devices and greater operators’ experience have certainly contributed to an increase in the efficacy and safety of the procedure over the years. Despite this, thromboembolic and bleeding complications after TAVI remain prevalent and affect morbidity and mortality. Indeed, TAVI interventions are associated with the occurrence of thrombotic and haemorrhagic events, which can occur periprocedurally or during the short- or long-term follow up after the index procedure.7,8 Given the observed important rise in the number of interventions, it is of utmost relevance to determine the optimal antithrombotic strategy after TAVI. In this scenario, an accurate assessment of the balance between thrombotic and bleeding risk, which can be augmented if an unnecessarily potent antithrombotic regimen is chosen, is critical. The aim of this article is to provide a brief and comprehensive overview of the current status of knowledge on antithrombotic treatment strategies following TAVI procedures, focusing on guidelines recommendations and the available evidence to support them, and of currently on-going trials that are evaluating different antithrombotic regimens in this scenario.
Adverse Events After Transcatheter Aortic Valve Implantation
Both thromboembolic and bleeding events after TAVI are major concerns due to their prognostic impact. These complications may be associated with procedural issues, but also with underlying risk factors in the long term, taking into consideration that these procedures are often performed in elderly and frail patients with several comorbidities.
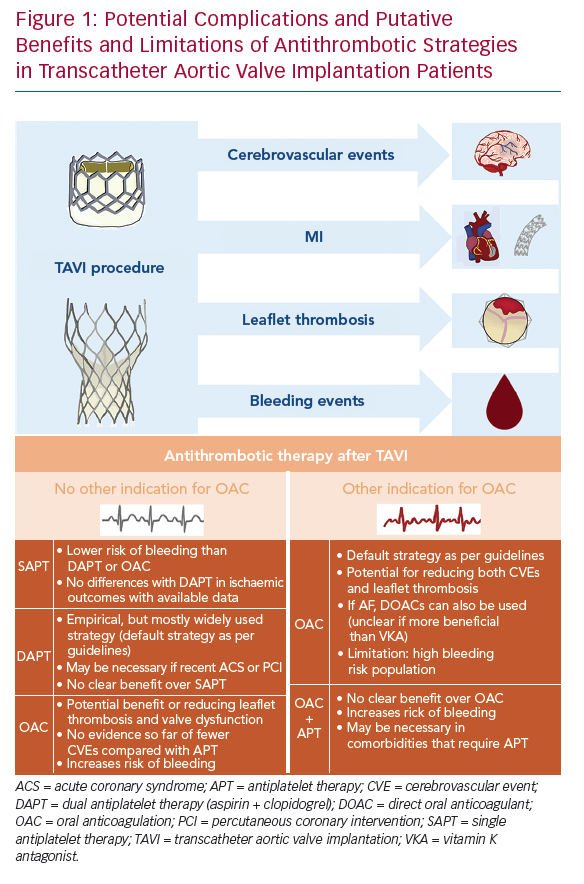
Early thromboembolic events can be considered mainly procedural, and due to device positioning and implantation. Periprocedural complications include cerebrovascular events (CVEs), systemic embolisation and MI. Of note, several factors and phenomena occurring during TAVI and in the early postprocedural period contribute to create a prothrombotic environment. These include: native leaflets are not removed and may be mechanically damaged during TAVI, which may lead to embolisation of small valve fragments and to the exposure in the bloodstream of molecules that help increasing thrombogenicity and inflammation (unlike normal valves, stenotic valve leaflets are rich in tissue factor and thrombin); changes in the flow patterns that may predispose to thrombus formation; and other mechanisms occurring during positioning and implantation (aortic wall injury, air embolism, rapid pacing or haemodynamic instability that may lead to cerebral hypoperfusion, etc.).7–10
CVEs are undoubtedly among the most feared complications of TAVI, since they have a considerable impact on morbidity and mortality.11–13 The greatest risk of suffering a clinically apparent stroke is within 24–48 hours after TAVI, but remains high for up to 2–3 months. The rates of CVEs (including transient ischaemic attacks and strokes) are highly variable among published studies, ranging from approximately 2–6% at 30 days, and reaching 7–9% at 1 year of follow-up.11,13 Acute events can be clearly attributed to procedural issues (i.e. device manipulation, repeated positioning or post-valve deployment balloon dilatation), whereas the main underlying cause of subacute (1–30 days) and late (>30 days) events is the presence of AF. The prevalence of AF among TAVI candidates (usually elderly patients) ranges approximately 20–50%, and is the major predictor of late CVEs in this population.14,15
In addition, 10–30% of patients develop new-onset AF after TAVI, which is less frequent with the transfemoral approach. Remarkably, new-onset AF is often undiagnosed, and it represents an important predictor of subacute CVEs. Overall, TAVI patients with AF have a high baseline cardioembolic risk, and both pre-existing and new-onset AF appear to be associated not only with CVEs, but also with mortality, although controversial results can be found in the scientific literature.14–17 Therefore, efforts should be made to properly diagnose and treat AF, which may frequently require chronic anticoagulation therapy, in this subset of patients.
The incidence of MI after TAVI is relatively low (1–2%) and mostly restricted to the periprocedural period, thus, it may reflect myocardial injury during the procedure. A higher degree of myocardial injury has been associated with increased mortality in some studies, but other investigations failed to observe a prognostic impact in TAVI patients.18–21 Nevertheless, it is relevant to identify coronary artery disease in TAVI candidates, since both conditions frequently coexist (30–70%) and the severity of coronary artery disease might impact prognosis after TAVI.22–24 The decision about revascularisation, its completeness and timing (staged or concomitant procedures) should be made on a case-by-case basis for patients undergoing TAVI. Regardless, a period of dual antiplatelet therapy (DAPT) after coronary revascularisation is mandatory, which has also evident implications in the decision-making process of selecting the antithrombotic treatment regimen after TAVI.25,26
The spectrum of bioprosthetic leaflet thrombosis comprises subclinical imaging findings, such as early reduced leaflet motion and hypoattenuated leaflet thickening, to clinically apparent valve thrombosis with elevated gradients and clinical manifestations (i.e. heart failure, valve dysfunction and stroke or systemic embolisms).27,28 Its pathogenesis is not completely known, although several mechanisms are involved, including surface, haemostatic and haemodynamic factors.29 The presence of hypoattenuated leaflet thickening and reduced leaflet motion is not infrequent after TAVI (up to 10–15% of cases).
A retrospective observational study reported that reduced leaflet motion could be associated with a higher risk of stroke.30 However, it remains uncertain whether these imaging abnormalities are clinically meaningful or just represent a subclinical finding. Fortunately, clinical valve thrombosis is an infrequent phenomenon with an estimated incidence of 0.03–0.07% per year,7 although a single-centre study reported an overall incidence of 2.8%, with a median time of diagnosis of approximately 6 months after the procedure.30 Of note, predictors of clinical valve thrombosis are the use of a balloon-expandable device (compared with self-expanding or mechanically expanded devices), a valve-in-valve procedure and treatment with antiplatelet agents alone.30 Interestingly, the use of oral anticoagulation (OAC) has consistently shown to be effective in reducing valve thickening and transvalvular gradients in the majority of cases of subclinical or clinical valve thrombosis, although the risk–benefit balance remains to be determined.28,30
Bleeding events also play a major role in short- and long-term prognosis after TAVI, and its severity can be categorised, for the sake of standardisation, according to the Valve Academic Research Consortium criteria.31 Indeed, early and late haemorrhagic events have a negative impact on cardiovascular mortality.32,33 Early (<30 days) major bleeding complications after TAVI are frequent (10–15%), and represent a strong independent predictor of early mortality.32 They are mostly due to vascular or access-site complications, with pericardial bleeding being less common.
The improvement in operators’ experience and in new generation devices (reduction in sheaths size) is leading to a reduction in the events related with the procedural technique. Remarkably, late (>30 days) bleeding events are associated with an important increase in mortality risk of more than threefold.33 Regarding late outcomes, non-access-site bleeding has a greater impact on prognosis, with gastrointestinal haemorrhages being the most frequent location. Importantly, late bleeding complications may be attributed to the baseline risk of patients undergoing TAVI, but antithrombotic regimens also play a role in this vulnerable population.34
Antithrombotic Therapy
There is clearly insufficient evidence nowadays regarding optimal adjunct antithrombotic therapy after TAVI, which is reflected in the large heterogeneity of drug regimens that are used in clinical practice.35 In fact, two different hypotheses – the antiplatelet and the antithrombin hypotheses – have been postulated to support the use of antiplatelet and anticoagulant agents, respectively. Unfortunately, it remains unclear whether thromboembolic complications after TAVI are mainly due to platelet- or thrombin-mediated clot formation. An accurate evaluation of the fine balance between a potential prevention of thromboembolic complications without unnecessarily increasing the risk of bleeding is crucial to determine the optimal antithrombotic therapy in this high-risk population (Figure 1). In this section, we summarise current guideline recommendations, most of them empirically based, and available evidence regarding the efficacy and safety of antiplatelet and anticoagulant agents after TAVI.
Guidelines Recommendations
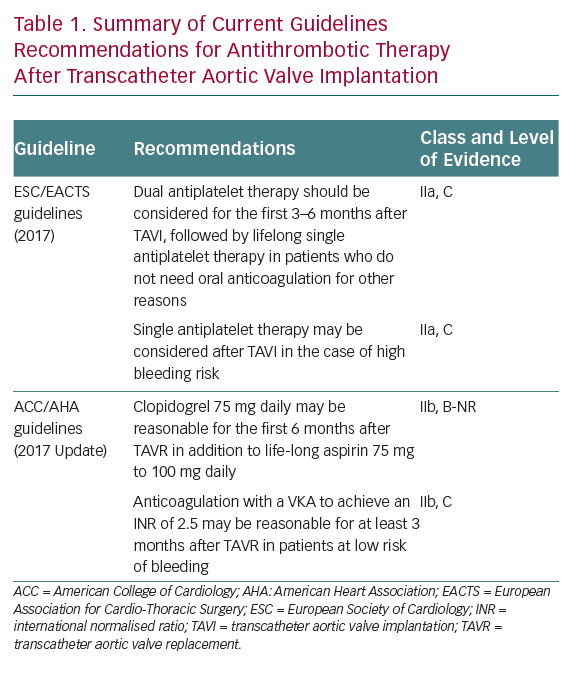
The recommendations of the European Society of Cardiology and American College of Cardiology/American Heart Association regarding antithrombotic therapy after TAVI are not uniform.1,2 This is far from surprising, as they are mostly based on relatively small non-randomised studies and expert consensus. Current guidelines recommendations on postprocedural antithrombotic strategies are summarised in Table 1.
European guidelines advocate the use of DAPT with aspirin and clopidogrel for the first 3–6 months, followed by lifelong single antiplatelet therapy (SAPT) as the default strategy for patients without any other indication for OAC, whereas SAPT may be considered for patients with high bleeding risk.1 US guidelines suggest DAPT with clopidogrel for the first 6 months in addition to lifelong aspirin, whereas OAC with a vitamin K antagonist (VKA) is proposed for at least 3 months after TAVI for patients at low risk of bleeding to diminish the risk of valve thrombosis.2 Both guidelines agree in recommending lifelong OAC for patients with other indications for OAC (the most frequent being AF), although no clear statement is provided with regards to the need for concomitant antiplatelet therapy in these patients.1,2
Antiplatelet Therapy: Available Evidence
The most widely adopted postprocedural antithrombotic regimen for TAVI patients, in the absence of an indication for OAC, is DAPT with aspirin (indefinitely) and clopidogrel (3–6 months). This strategy is empirical and based, first and foremost, on current recommendations for patients undergoing percutaneous coronary interventions.25 Further, anatomopathological analyses have suggested that neointimal tissue invasion and full endothelialisation of the valve stent frame occur approximately 3 months after the procedure, with a decrease in thromboembolic events thereafter, which might support this antithrombotic strategy.36 Recent evidence, however, has questioned the usefulness and safety of DAPT over SAPT with aspirin.
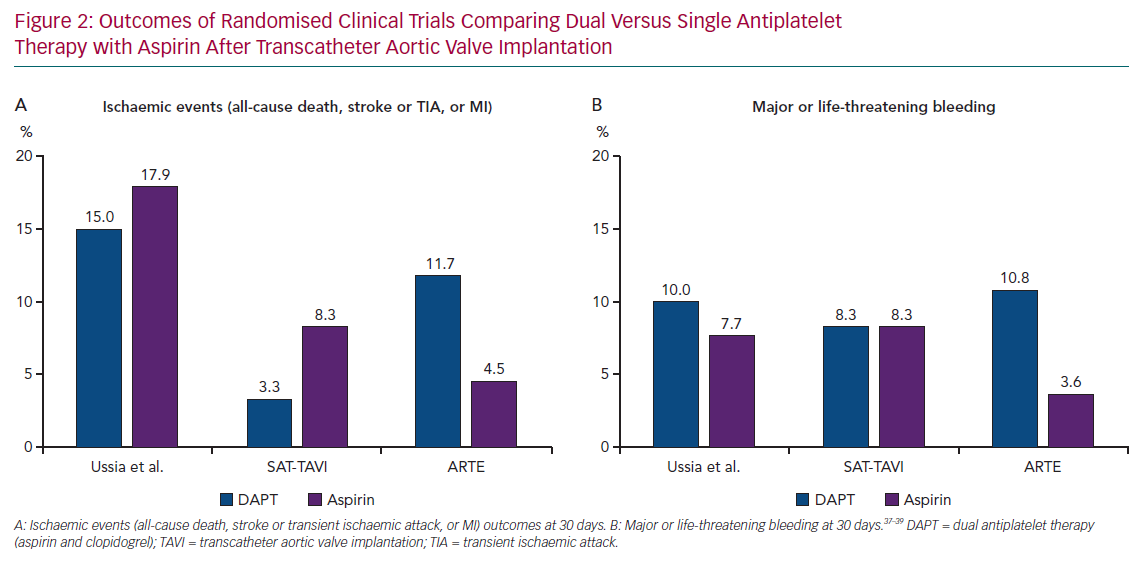
Three small randomised trials have compared DAPT (3 or 6 months) with aspirin monotherapy after TAVI (Figure 2).37–39 None of them found a significant benefit of DAPT in terms of the prevention of thromboembolic events, although the studies were clearly not powered to detect differences in ischaemic outcomes due to their small sample size.
The Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation (ARTE) trial was the largest of these studies. ARTE compared 3 months of DAPT with SAPT plus aspirin in 222 patients with no indication for OAC after implantation of a balloon-expandable device, although it was stopped prematurely due to funding issues and slow recruitment.39 The net primary endpoint (death, MI, stroke or transient ischaemic attack, or major or life-threatening bleeding) numerically tended to occur more frequently in the DAPT group (15.3% versus 7.2%; p=0.065) at 3 months after the procedure. Of note, no statistically significant differences were observed in individual ischaemic endpoints or mortality, whereas DAPT was associated with a greater rate of major or life-threatening bleeding events compared with SAPT (10.8% versus 3.6%; p=0.038).39
This was corroborated in a patient-level meta-analysis of these three randomised trials (n=421), in which DAPT was associated with a higher risk of major or life-threatening bleeding events compared with aspirin monotherapy at 30 days of follow up (11.4% versus 5.2%; OR 2.24; 95% CI [1.12–4.46]; p=0.022) without showing any beneficial effect on ischaemic events.40
A recently published mechanistic investigation showed a better pharmacodynamic efficacy of ticagrelor compared with clopidogrel (both in association with aspirin) in TAVI patients with suboptimal response to clopidogrel, as measured with the VerifyNow PRUTest (Werfen). The study was not powered to evaluate differences in clinical endpoints, and the high level of platelet inhibition achieved with ticagrelor should actually suggest caution before prescribing a potent antiplatelet agent in this elderly population with a high risk of bleeding. Nevertheless, an interesting reflection can be drawn from this trial, as a very high percentage of suboptimal responders to clopidogrel (>70%) was observed and, thus, it questions again the usefulness of the addition of clopidogrel to aspirin in this setting.41
Overall, cumulative data from observational and small randomised investigations considered together do not support current common practice of DAPT after TAVI, as it has not been proven superior in reducing thromboembolic events compared with SAPT with aspirin, which could be a safer and similarly effective option.42 Some on-going trials that are mentioned below are presently testing this hypothesis and will provide important insights on the subject.
Anticoagulant Therapy: Available Evidence
The above-mentioned effectiveness of OAC in preventing and treating subclinical and clinical leaflet thrombosis that has been reported in observational studies has provided the rationale for suggesting an OAC-based strategy after TAVI, as recommended in the US guidelines.2,28,30 However, the evidence supporting this recommendation is weak and several doubts may arise, such as the duration of OAC that should be prescribed, the actual prognostic relevance of subclinical leaflet thrombosis or, more importantly, the impact on haemorrhagic events.
Interestingly, in a recent report of the large nationwide FRANCE-TAVI registry, OAC at discharge (administered to >70% of cases due to AF) was independently associated with a lower risk of valve dysfunction and, conversely, with a higher risk of all-cause mortality.43 Indeed, the uncertainty of the trade-off between the potential benefit of preventing an infrequent entity, such as clinical valve thrombosis, and the potential harm of prolonged OAC in patients without AF precludes the use of long-term OAC as a default strategy for patients undergoing TAVI.
The management of antithrombotic therapy after TAVI for patients with other indications for long-term OAC, with AF being the most frequent aetiology, is particularly challenging. As commented previously, the presence of AF is very common in patients undergoing TAVI, and is associated with an increased risk of cerebrovascular events and mortality.14,17 Of note, the addition of antiplatelet therapy to OAC does not appear to reduce the incidence of thromboembolic events and actually increases the risk of major or life-threatening bleeding.44,45 Hence, if no other reason for associating antiplatelet agents is present (e.g. recent percutaneous coronary intervention), available evidence suggests that OAC alone without concomitant antiplatelet therapy may be the preferred antithrombotic treatment for TAVI patients with AF.
The use of direct oral anticoagulants (DOACs) appears particularly attractive due to their favourable safety profile when compared with VKA, particularly for patients at high risk of bleeding or if combination with antiplatelet drugs is required.46,47 However, the evidence regarding the usefulness of DOACs after TAVI is extremely scarce to date, and is mostly based on small subgroup analyses of observational studies. According to available data, DOACs may have a similar efficacy as VKA in preventing thromboembolic events after TAVI, and also for treatment of subclinical leaflet thrombosis.28,48
A small study even suggested that apixaban might confer a benefit (composite endpoint at 30 days of all-cause mortality, all stroke, life-threatening bleeding, acute kidney injury, coronary obstruction, major vascular complications and valve dysfunction requiring re-intervention) compared with VKA for patients with AF after transfemoral TAVI.49 Conversely, a recently published multicentre registry evaluating the impact of the type of anticoagulant agent for TAVI patients (n=962) in need of OAC therapy showed a higher risk of ischaemic events (composite endpoint of all-cause mortality, MI and any cerebrovascular event) with the use of DOACs compared with VKA at 1 year of follow up without noticing differences in bleeding risk.50 Hence, further investigations are warranted to determine the true usefulness of DOACs in this scenario and, in fact, several on-going studies are evaluating the efficacy and safety of DOACs for TAVI patients with and without other indications for OAC.
On-going Studies
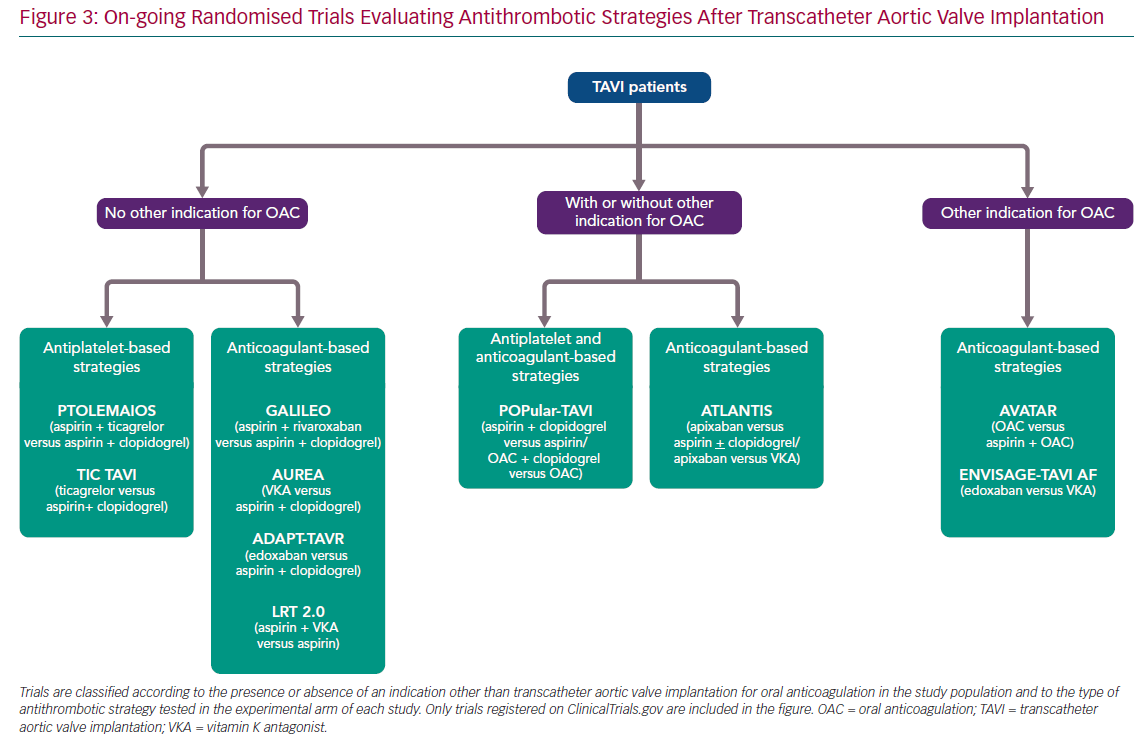
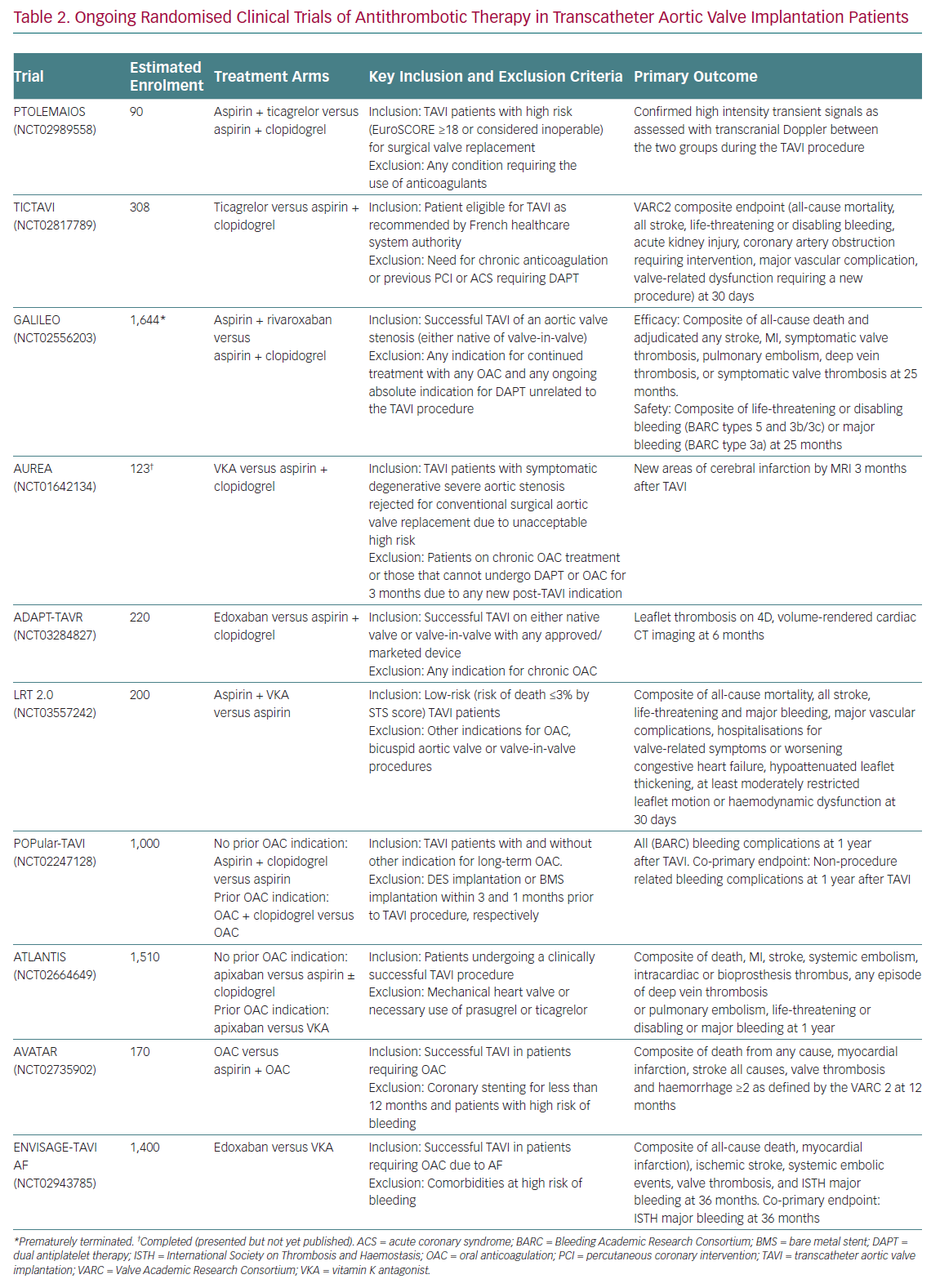
Current recommendations of practice guidelines regarding antithrombotic therapy after TAVI, as previously mentioned, are not fully evidence based. However, this is a very active field of research with several on-going randomised trials (Figure 3). A detailed description of these investigations is beyond the scope of this article, but it is worthwhile commenting briefly on some of them due to their special interest (Table 2).
The Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation (POPular-TAVI; NCT02247128) trial will provide important insights into the management of antithrombotic treatment for TAVI patients. POPular-TAVI will assess the safety in terms of bleeding (primary endpoint) and the efficacy of the following regimens: aspirin monotherapy compared with DAPT with aspirin plus clopidogrel in a cohort of patients without an indication for OAC prior to TAVI (n=684); and OAC alone compared with OAC plus clopidogrel in a cohort of patients with an indication for OAC prior to TAVI (n=316).
In patients without other indications for OAC, some small studies are evaluating the possible benefit of ticagrelor in this scenario, either in association with aspirin, in the Trial to Assess the Safety and Efficacy of Prophylactic TicagrelOr With Acetylsalicylic Acid Versus CLopidogrel With Acetylsalicylic Acid in the Development of Cerebrovascular EMbolic Events During TAVI (PTOLEMAIOS; NCT02989558) trial, or in monotherapy in the Safety Profile Evaluation of TICagrelor Alone Compared to a Combination of Lysine Acetylsalicylate-Clopidogrel in the Context of Transcatheter Aortic Valve Implantation (TICTAVI; NCT02817789) study.
Several studies are trying to assess the usefulness of anticoagulation-based strategies in TAVI patients without any other known indication for OAC. Of note, the Global Study Comparing a rivAroxaban-based Antithrombotic Strategy to an antipLatelet-based Strategy After Transcatheter aortIc vaLve rEplacement to Optimize Clinical Outcomes (GALILEO; NCT02556203) trial, which was evaluating the usefulness of a reduced dose of rivaroxaban (10 mg daily) in association with aspirin compared with standard DAPT, had to be prematurely terminated after enrolling 1,644 patients due to increased risks of all-cause mortality, thromboembolic events and bleeding in the rivaroxaban arm. The study has been recently published and its findings evidently cast doubts on the need for anticoagulant therapy in TAVI patients without any other indication for OAC (e.g. AF, mechanic valves, etc.).51
Other small-scale investigations will provide more information into the matter, such as the Dual Antiplatelet Therapy Versus Oral Anticoagulation for a Short Time to Prevent Cerebral Embolism After TAVI (AUREA; NCT01642134) and the Anticoagulant Versus Dual Antiplatelet Therapy for Preventing Leaflet Thrombosis and Cerebral Embolisation After Transcatheter Aortic Valve Replacement (ADAPT-TAVR; NCT03284827) trials, which are comparing the effectiveness of VKA and edoxaban in monotherapy, respectively, versus standard DAPT with aspirin and clopidogrel. Of note, the results of the AUREA trial have been recently reported (but not published at the time this manuscript was written), showing that OAC with VKA failed to reduce the incidence of new subclinical ischaemic cerebral lesions, assessed by magnetic resonance imaging at 6 days and 3 months after TAVI, when compared with DAPT.
Another small study, the Strategies to Prevent Transcatheter Heart Valve Dysfunction in Low Risk Transcatheter Aortic Valve Replacement (LRT 2.0; NCT03557242), is evaluating the effect of adding VKA to aspirin compared with aspirin monotherapy.
Two relevant large trials are presently exploring the usefulness of DOACs following TAVI: the Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis (ATLANTIS; NCT02664649) study is evaluating the efficacy and safety of full-dose apixaban 5 mg twice daily (dose adjustment according to the drug label) compared either with VKA in patients with an indication other than TAVI for OAC or with standard of care antiplatelet therapy (DAPT or SAPT) in subjects without OAC indication; and the Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients with Atrial Fibrillation (ENVISAGE TAVI AF; NCT02943785) trial, which is comparing an edoxaban-based regimen with a VKA-based regimen in AF patients with indication for OAC after TAVI.
For patients with a concomitant indication for anticoagulation, the value, if any, of adding aspirin to OAC (VKA or DOAC) compared with OAC alone is being tested in the Anticoagulation Alone Versus Anticoagulation and Aspirin Following Transcatheter Aortic Valve Interventions (AVATAR; NCT02735902) trial. Finally, a significant proportion of AF patients undergoing TAVI is at increased risk of bleeding complications and, thus, may also have an absolute or relative contraindication for OAC. In this population, a non-pharmacological approach with concomitant TAVI and left atrial appendage occlusion could be an attractive option. The efficacy and safety of this strategy is being evaluated in the WATCHMAN for Patients With Atrial Fibrillation Undergoing Transcatheter Aortic Valve Replacement (WATCH-TAVR; NCT03173534) and the Comparison of Left Atrial Appendage Occlusion Versus Standard Medical Therapy in Patients in AF Undergoing TAVI (TAVI/LAAO; NCT03088098) trials.
Conclusion
The introduction of TAVI has represented an important breakthrough in cardiology and has changed the clinical practice for patients with aortic stenosis. Since the number of procedures is growing, it is crucial to determine the optimal antithrombotic therapy after TAVI, which to date remains a challenge due to the scarce available evidence. DAPT with aspirin and clopidogrel is the most commonly used strategy for patients without other indications for OAC, although recent evidence has questioned this approach, suggesting that SAPT with aspirin may be sufficient and less harmful in terms of bleeding in this setting. For patients with an indication for OAC other than TAVI (mostly AF), monotherapy with OAC appears to be the most suitable strategy. In the absence of contraindications, the use of DOACs in this scenario is appealing due to their better safety profile compared with VKA, but this hypothesis needs to be confirmed by dedicated studies. The results of several on-going trials will provide interesting insights on the subject in the upcoming years.